Genetic dissection of the pluripotent proteome through multi-omics data integration
- PMID: 37082146
- PMCID: PMC10112288
- DOI: 10.1016/j.xgen.2023.100283
Genetic dissection of the pluripotent proteome through multi-omics data integration
Abstract
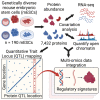
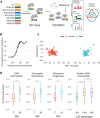
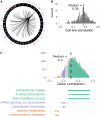
Genetic background drives phenotypic variability in pluripotent stem cells (PSCs). Most studies to date have used transcript abundance as the primary molecular readout of cell state in PSCs. We performed a comprehensive proteogenomics analysis of 190 genetically diverse mouse embryonic stem cell (mESC) lines. The quantitative proteome is highly variable across lines, and we identified pluripotency-associated pathways that were differentially activated in the proteomics data that were not evident in transcriptome data from the same lines. Integration of protein abundance to transcript levels and chromatin accessibility revealed broad co-variation across molecular layers as well as shared and unique drivers of quantitative variation in pluripotency-associated pathways. Quantitative trait locus (QTL) mapping localized the drivers of these multi-omic signatures to genomic hotspots. This study reveals post-transcriptional mechanisms and genetic interactions that underlie quantitative variability in the pluripotent proteome and provides a regulatory map for mESCs that can provide a basis for future mechanistic studies.
Keywords: chromatin accessibility; diversity outbred mice; eQTL; embryonic stem cells; ground state metastability; multi-omics factor analysis; pQTL; pluripotency; proteomics; transcriptomics.
© 2023 The Author(s).
Conflict of interest statement
T.C. has an equity interest in Predictive Biology, Inc.
Figures



References
-
- Skelly D.A., Czechanski A., Byers C., Aydin S., Spruce C., Olivier C., Choi K., Gatti D.M., Raghupathy N., Keele G.R., et al. Mapping the effects of genetic variation on chromatin state and gene expression reveals loci that control ground state pluripotency. Cell Stem Cell. 2020;27:459–469.e8. doi: 10.1016/j.stem.2020.07.005. - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

