GCK exonic mutations induce abnormal biochemical activities and result in GCK-MODY
- PMID: 37082200
- PMCID: PMC10110986
- DOI: 10.3389/fgene.2023.1120153
GCK exonic mutations induce abnormal biochemical activities and result in GCK-MODY
Abstract
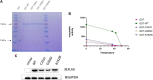
Objective: Glucokinase-maturity-onset diabetes of the young (GCK-MODY; MODY2) is a rare genetic disorder caused by mutations in the glucokinase (GCK) gene. It is often under- or misdiagnosed in clinical practice, but correct diagnosis can be facilitated by genetic testing. In this study, we examined the genes of three patients diagnosed with GCK-MODY and tested their biochemical properties, such as protein stability and half-life, to explore the function of the mutant proteins and identify the pathogenic mechanism of GCK-MODY. Methods: Three patients with increased blood glucose levels were diagnosed with MODY2 according to the diagnostic guidelines of GCK-MODY proposed by the International Society for Pediatric and Adolescent Diabetes (ISPAD) in 2018. Next-generation sequencing (whole exome detection) was performed to detect gene mutations. The GCK gene and its mutations were introduced into the pCDNA3.0 and pGEX-4T-1 vectors. Following protein purification, enzyme activity assay, and protein immunoblotting, the enzyme activity of GCK was determined, along with the ubiquitination level of the mutant GCK protein. Results: Genetic testing revealed three mutations in the GCK gene of the three patients, including c.574C>T (p.R192W), c.758G>A (p.C253Y), and c.794G>A (p.G265D). The biochemical characteristics of the protein encoded by wild-type GCK and mutant GCK were different, compared to wild-type GCK, the enzyme activity encoded by the mutant GCK was reduced, suggesting thermal instability of the mutant GST-GCK. The protein stability and expression levels of the mutant GCK were reduced, and the enzyme activity of GCK was negatively correlated with the levels of fasting blood glucose and HbA1c. In addition, ubiquitination of the mutant GCK protein was higher than that of the wild-type, suggesting a higher degradation rate of mutant GCK than WT-GCK. Conclusion: GCK mutations lead to changes in the biochemical characteristics of its encoded proteins. The enzyme activities, protein expression, and protein stability of GCK may be reduced in patients with GCK gene mutations, which further causes glucose metabolism disorders and induces MODY2.
Keywords: GCK; GCK-MODY; biochemical activity; gene variants; glucokinase.
Copyright © 2023 Dai, Yang, Zhang, Ma, Chen, Zhang, Lv, Li, Tang, Zhen, Lu, Li, Hu, Xiao and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Genetic and clinical characteristics of Chinese children with Glucokinase-maturity-onset diabetes of the young (GCK-MODY).BMC Pediatr. 2018 Mar 6;18(1):101. doi: 10.1186/s12887-018-1060-8. BMC Pediatr. 2018. PMID: 29510678 Free PMC article.
-
Two novel GCK mutations in Chinese patients with maturity-onset diabetes of the young.Endocrine. 2024 Jan;83(1):92-98. doi: 10.1007/s12020-023-03509-1. Epub 2023 Oct 17. Endocrine. 2024. PMID: 37847371
-
Diagnostic screening of MODY2/GCK mutations in the Norwegian MODY Registry.Pediatr Diabetes. 2008 Oct;9(5):442-9. doi: 10.1111/j.1399-5448.2008.00399.x. Epub 2008 Apr 9. Pediatr Diabetes. 2008. PMID: 18399931
-
Recognition and Management of Individuals With Hyperglycemia Because of a Heterozygous Glucokinase Mutation.Diabetes Care. 2015 Jul;38(7):1383-92. doi: 10.2337/dc14-2769. Diabetes Care. 2015. PMID: 26106223 Review.
-
Clinical implications of the glucokinase impaired function - GCK MODY today.Physiol Res. 2020 Dec 22;69(6):995-1011. doi: 10.33549/physiolres.934487. Epub 2020 Nov 2. Physiol Res. 2020. PMID: 33129248 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

