A simplified protocol for the generation of cortical brain organoids
- PMID: 37082206
- PMCID: PMC10110973
- DOI: 10.3389/fncel.2023.1114420
A simplified protocol for the generation of cortical brain organoids
Abstract
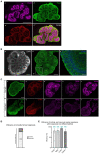
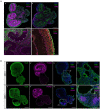
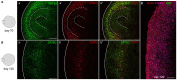
Human brain organoid technology has the potential to generate unprecedented insight into normal and aberrant brain development. It opens up a developmental time window in which the effects of gene or environmental perturbations can be experimentally tested. However, detection sensitivity and correct interpretation of phenotypes are hampered by notable batch-to-batch variability and low reproducibility of cell and regional identities. Here, we describe a detailed, simplified protocol for the robust and reproducible generation of brain organoids with cortical identity from feeder-independent induced pluripotent stem cells (iPSCs). This self-patterning approach minimizes media supplements and handling steps, resulting in cortical brain organoids that can be maintained over prolonged periods and that contain radial glial and intermediate progenitors, deep and upper layer neurons, and astrocytes.
Keywords: cortical brain organoids; dorsal identity; feeder-independent; human brain development; induced pluripotent stem cells; self-patterning.
Copyright © 2023 Eigenhuis, Somsen, van der Kroeg, Smeenk, Korporaal, Kushner, de Vrij and van den Berg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Robust and Highly Reproducible Generation of Cortical Brain Organoids for Modelling Brain Neuronal Senescence In Vitro.J Vis Exp. 2022 May 5;(183). doi: 10.3791/63714. J Vis Exp. 2022. PMID: 35604169
-
Novel model of cortical-meningeal organoid co-culture system improves human cortical brain organoid cytoarchitecture.Sci Rep. 2023 May 14;13(1):7809. doi: 10.1038/s41598-023-35077-9. Sci Rep. 2023. PMID: 37183210 Free PMC article.
-
Generation of Neurosphere-Derived Organoid-Like-Aggregates (NEDAS) from Neural Stem Cells.Curr Protoc. 2021 Feb;1(2):e15. doi: 10.1002/cpz1.15. Curr Protoc. 2021. PMID: 33534198
-
Cortical Organoids to Model Microcephaly.Cells. 2022 Jul 7;11(14):2135. doi: 10.3390/cells11142135. Cells. 2022. PMID: 35883578 Free PMC article. Review.
-
A beginner's guide on the use of brain organoids for neuroscientists: a systematic review.Stem Cell Res Ther. 2023 Apr 15;14(1):87. doi: 10.1186/s13287-023-03302-x. Stem Cell Res Ther. 2023. PMID: 37061699 Free PMC article.
Cited by
-
Human Brain Organoids: Development and Applications.J Microbiol Biotechnol. 2025 May 28;35:e2411040. doi: 10.4014/jmb.2411.11040. J Microbiol Biotechnol. 2025. PMID: 40443221 Free PMC article. Review.
-
Development of brain organoid technology derived from iPSC for the neurodegenerative disease modelling: a glance through.Front Mol Neurosci. 2023 Aug 3;16:1173433. doi: 10.3389/fnmol.2023.1173433. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37602192 Free PMC article. Review.
-
Brain Organoid Transplantation: A Comprehensive Guide to the Latest Advances and Practical Applications-A Systematic Review.Cells. 2025 Jul 14;14(14):1074. doi: 10.3390/cells14141074. Cells. 2025. PMID: 40710327 Free PMC article. Review.
-
Brain organoid as a model to study the role of mitochondria in neurodevelopmental disorders: achievements and weaknesses.Front Cell Neurosci. 2024 Jun 24;18:1403734. doi: 10.3389/fncel.2024.1403734. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38978706 Free PMC article. Review.
-
Altered neuroepithelial morphogenesis and migration defects in iPSC-derived cerebral organoids and 2D neural stem cells in familial bipolar disorder.Oxf Open Neurosci. 2024 Apr 3;3:kvae007. doi: 10.1093/oons/kvae007. eCollection 2024. Oxf Open Neurosci. 2024. PMID: 38638145 Free PMC article.
References
-
- Bruno S., Darzynkiewicz Z. (1992). Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 25, 31–40. - PubMed
LinkOut - more resources
Full Text Sources

