Comprehensive ex vivo and in vivo preclinical evaluation of novel chemo enzymatic decellularized peripheral nerve allografts
- PMID: 37082209
- PMCID: PMC10111265
- DOI: 10.3389/fbioe.2023.1162684
Comprehensive ex vivo and in vivo preclinical evaluation of novel chemo enzymatic decellularized peripheral nerve allografts
Abstract
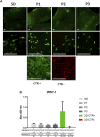
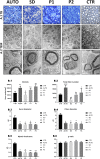
As a reliable alternative to autografts, decellularized peripheral nerve allografts (DPNAs) should mimic the complex microstructure of native nerves and be immunogenically compatible. Nevertheless, there is a current lack of decellularization methods able to remove peripheral nerve cells without significantly altering the nerve extracellular matrix (ECM). The aims of this study are firstly to characterize ex vivo, in a histological, biochemical, biomechanical and ultrastructural way, three novel chemical-enzymatic decellularization protocols (P1, P2 and P3) in rat sciatic nerves and compared with the Sondell classic decellularization method and then, to select the most promising DPNAs to be tested in vivo. All the DPNAs generated present an efficient removal of the cellular material and myelin, while preserving the laminin and collagen network of the ECM (except P3) and were free from any significant alterations in the biomechanical parameters and biocompatibility properties. Then, P1 and P2 were selected to evaluate their regenerative effectivity and were compared with Sondell and autograft techniques in an in vivo model of sciatic defect with a 10-mm gap, after 15 weeks of follow-up. All study groups showed a partial motor and sensory recovery that were in correlation with the histological, histomorphometrical and ultrastructural analyses of nerve regeneration, being P2 the protocol showing the most similar results to the autograft control group.
Keywords: acellular graft; decellularization; decellularized nerve allograft; peripheral nerve repair; tissue engineering.
Copyright © 2023 García-García, El Soury, Campos, Sánchez-Porras, Geuna, Alaminos, Gambarotta, Chato-Astrain, Raimondo and Carriel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Detergent-based decellularized peripheral nerve allografts: An in vivo preclinical study in the rat sciatic nerve injury model.J Tissue Eng Regen Med. 2020 Jun;14(6):789-806. doi: 10.1002/term.3043. Epub 2020 Apr 22. J Tissue Eng Regen Med. 2020. PMID: 32293801
-
Comparison of Decellularization Protocols to Generate Peripheral Nerve Grafts: A Study on Rat Sciatic Nerves.Int J Mol Sci. 2021 Feb 27;22(5):2389. doi: 10.3390/ijms22052389. Int J Mol Sci. 2021. PMID: 33673602 Free PMC article.
-
Qualitative and Quantitative Evaluation of a Novel Detergent-Based Method for Decellularization of Peripheral Nerves.Ann Biomed Eng. 2018 Nov;46(11):1921-1937. doi: 10.1007/s10439-018-2082-y. Epub 2018 Jul 9. Ann Biomed Eng. 2018. PMID: 29987538
-
Evaluation methods as quality control in the generation of decellularized peripheral nerve allografts.J Neural Eng. 2018 Apr;15(2):021003. doi: 10.1088/1741-2552/aaa21a. J Neural Eng. 2018. PMID: 29244032 Review.
-
Nerve regeneration using decellularized tissues: challenges and opportunities.Front Neurosci. 2023 Oct 19;17:1295563. doi: 10.3389/fnins.2023.1295563. eCollection 2023. Front Neurosci. 2023. PMID: 37928728 Free PMC article. Review.
Cited by
-
Mechanisms underlying the cell-matrixed nerve grafts repairing peripheral nerve defects.Bioact Mater. 2023 Sep 17;31:563-577. doi: 10.1016/j.bioactmat.2023.09.002. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37753326 Free PMC article.
-
Comparison of Printable Biomaterials for Use in Neural Tissue Engineering: An In Vitro Characterization and In Vivo Biocompatibility Assessment.Polymers (Basel). 2024 May 17;16(10):1426. doi: 10.3390/polym16101426. Polymers (Basel). 2024. PMID: 38794619 Free PMC article.
-
Exploring an innovative decellularization protocol for porcine nerve grafts: a translational approach to peripheral nerve repair.Front Neuroanat. 2024 Mar 19;18:1380520. doi: 10.3389/fnana.2024.1380520. eCollection 2024. Front Neuroanat. 2024. PMID: 38567289 Free PMC article.
References
-
- Campos F., Bonhome-Espinosa A. B., Carmona R., Duran J. D. G., Kuzhir P., Alaminos M., et al. (2021). In vivo time-course biocompatibility assessment of biomagnetic nanoparticles-based biomaterials for tissue engineering applications. Mater Sci. Eng. C Mater Biol. Appl. 118, 111476. 10.1016/j.msec.2020.111476 - DOI - PubMed
-
- Campos F., Bonhome-Espinosa A. B., Chato-Astrain J., Sanchez-Porras D., Garcia-Garcia O. D., Carmona R., et al. (2020). Evaluation of fibrin-agarose tissue-like hydrogels biocompatibility for tissue engineering applications. Front. Bioeng. Biotechnol. 8, 596. 10.3389/fbioe.2020.00596 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

