MEPIRAPIM-derived synthetic cannabinoids inhibit T-type calcium channels with divergent effects on seizures in rodent models of epilepsy
- PMID: 37082241
- PMCID: PMC10110893
- DOI: 10.3389/fphys.2023.1086243
MEPIRAPIM-derived synthetic cannabinoids inhibit T-type calcium channels with divergent effects on seizures in rodent models of epilepsy
Abstract
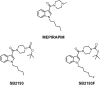
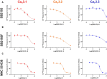
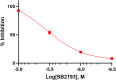
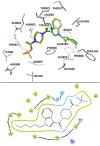
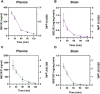
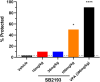
Background: T-type Ca2+ channels (Cav3) represent emerging therapeutic targets for a range of neurological disorders, including epilepsy and pain. To aid the development and optimisation of new therapeutics, there is a need to identify novel chemical entities which act at these ion channels. A number of synthetic cannabinoid receptor agonists (SCRAs) have been found to exhibit activity at T-type channels, suggesting that cannabinoids may provide convenient chemical scaffolds on which to design novel Cav3 inhibitors. However, activity at cannabinoid type 1 (CB1) receptors can be problematic because of central and peripheral toxicities associated with potent SCRAs. The putative SCRA MEPIRAPIM and its analogues were recently identified as Cav3 inhibitors with only minimal activity at CB1 receptors, opening the possibility that this scaffold may be exploited to develop novel, selective Cav3 inhibitors. Here we present the pharmacological characterisation of SB2193 and SB2193F, two novel Cav3 inhibitors derived from MEPIRAPIM. Methods: The potency of SB2193 and SB2193F was evaluated in vitro using a fluorometric Ca2+ flux assay and confirmed using whole-cell patch-clamp electrophysiology. In silico docking to the cryo-EM structure of Cav3.1 was also performed to elucidate structural insights into T-type channel inhibition. Next, in vivo pharmacokinetic parameters in mouse brain and plasma were determined using liquid chromatography-mass spectroscopy. Finally, anticonvulsant activity was assayed in established genetic and electrically-induced rodent seizure models. Results: Both MEPIRAPIM derivatives produced potent inhibition of Cav3 channels and were brain penetrant, with SB2193 exhibiting a brain/plasma ratio of 2.7. SB2193 was further examined in mouse seizure models where it acutely protected against 6 Hz-induced seizures. However, SB2193 did not reduce spontaneous seizures in the Scn1a +/- mouse model of Dravet syndrome, nor absence seizures in the Genetic Absence Epilepsy Rat from Strasbourg (GAERS). Surprisingly, SB2193 appeared to increase the incidence and duration of spike-and-wave discharges in GAERS animals over a 4 h recording period. Conclusion: These results show that MEPIRAPIM analogues provide novel chemical scaffolds to advance Cav3 inhibitors against certain seizure types.
Keywords: 6 Hz; Cav3 channels; Dravet syndrome; GAERS; cannabinoid; epilepsy; mepirapim.
Copyright © 2023 Harman, Udoh, McElroy, Anderson, Kevin, Banister, Ametovski, Markham, Bladen, Doohan, Greba, Laprairie, Snutch, McGregor, Howland and Arnold.
Conflict of interest statement
JA, RL, and IM have served as expert witnesses in various medicolegal cases involving cannabis and cannabinoids. JA, LA, and IM. hold patents on cannabinoid therapies (PCT/AU2018/05089 and PCT/AU2019/050554). JA has received consulting fees from Creo Inc. and Medicinal Cannabis Industry Australia (MCIA). RL acts as a consultant to Shackleford Pharma Inc. IM acts as a consultant to Kinoxis Therapeutics and has received honoraria from Janssen. He has also received consulting fees from MCIA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Putative Synthetic Cannabinoids MEPIRAPIM, 5F-BEPIRAPIM (NNL-2), and Their Analogues Are T-Type Calcium Channel (CaV3) Inhibitors.ACS Chem Neurosci. 2022 May 4;13(9):1395-1409. doi: 10.1021/acschemneuro.1c00822. Epub 2022 Apr 20. ACS Chem Neurosci. 2022. PMID: 35442021
-
Cannabinoid-like compounds found in non-cannabis plants exhibit antiseizure activity in genetic mouse models of drug-resistant epilepsy.Epilepsia. 2025 Jan;66(1):303-314. doi: 10.1111/epi.18177. Epub 2024 Nov 12. Epilepsia. 2025. PMID: 39530840
-
Modulation of human T-type calcium channels by synthetic cannabinoid receptor agonists in vitro.Neuropharmacology. 2021 Apr 1;187:108478. doi: 10.1016/j.neuropharm.2021.108478. Epub 2021 Feb 16. Neuropharmacology. 2021. PMID: 33600843
-
Voltage-Gated Calcium Channels in Epilepsy.In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. PMID: 22787663 Free Books & Documents. Review.
-
Spike-wave discharges in adult Sprague-Dawley rats and their implications for animal models of temporal lobe epilepsy.Epilepsy Behav. 2014 Mar;32:121-31. doi: 10.1016/j.yebeh.2014.01.004. Epub 2014 Feb 15. Epilepsy Behav. 2014. PMID: 24534480 Free PMC article. Review.
References
-
- Anderson L. L., Heblinski M., Absalom N. L., Hawkins N. A., Bowen M. T., Benson M. J., et al. (2021). Cannabigerolic acid, a major biosynthetic precursor molecule in cannabis, exhibits divergent effects on seizures in mouse models of epilepsy. Br. J. Pharmacol. 178, 4826–4841. 10.1111/bph.15661 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

