C-type natriuretic peptide (CNP) in the paraventricular nucleus-mediated renal sympatho-inhibition
- PMID: 37082246
- PMCID: PMC10110992
- DOI: 10.3389/fphys.2023.1162699
C-type natriuretic peptide (CNP) in the paraventricular nucleus-mediated renal sympatho-inhibition
Abstract
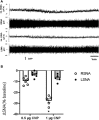
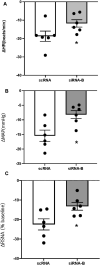
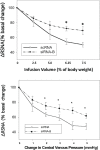
Volume reflex produces sympatho-inhibition that is mediated by the hypothalamic paraventricular nucleus (PVN). However, the mechanisms for the sympatho-inhibitory role of the PVN and the neurochemical factors involved remain to be identified. In this study, we proposed C-type natriuretic peptide (CNP) as a potential mediator of this sympatho-inhibition within the PVN. Microinjection of CNP (1.0 μg) into the PVN significantly decreased renal sympathetic nerve activity (RSNA) (-25.8% ± 1.8% vs. -3.6% ± 1.5%), mean arterial pressure (-15.0 ± 1.9 vs. -0.1 ± 0.9 mmHg) and heart rate (-23.6 ± 3.5 vs. -0.3 ± 0.9 beats/min) compared with microinjection of vehicle. Picoinjection of CNP significantly decreased the basal discharge of extracellular single-unit recordings in 5/6 (83%) rostral ventrolateral medulla (RVLM)-projecting PVN neurons and in 6/13 (46%) of the neurons that were not antidromically activated from the RVLM. We also observed that natriuretic peptide receptor type C (NPR-C) was present on the RVLM projecting PVN neurons detected by dual-labeling with retrograde tracer. Prior NPR-C siRNA microinjection into the PVN significantly blunted the decrease in RSNA to CNP microinjections into the PVN. Volume expansion-mediated reduction in RSNA was significantly blunted by prior administration of NPR-C siRNA into the PVN. These results suggest a potential role for CNP within the PVN in regulating RSNA, specifically under physiological conditions of alterations in fluid balance.
Keywords: C-type natriuretic peptide; central nervous system; paraventricular nucleus; renal sympathetic nerve activity; volume reflex.
Copyright © 2023 Zheng, Patel, Liu and Patel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Sympathoexcitatory input from hypothalamic paraventricular nucleus neurons projecting to rostral ventrolateral medulla is enhanced after myocardial infarction.Am J Physiol Heart Circ Physiol. 2020 Dec 1;319(6):H1197-H1207. doi: 10.1152/ajpheart.00273.2020. Epub 2020 Sep 18. Am J Physiol Heart Circ Physiol. 2020. PMID: 32946261
-
Sympathoexcitation by hypothalamic paraventricular nucleus neurons projecting to the rostral ventrolateral medulla.J Physiol. 2018 Oct;596(19):4581-4595. doi: 10.1113/JP276223. Epub 2018 Aug 18. J Physiol. 2018. PMID: 30019338 Free PMC article.
-
Salusin-β in paraventricular nucleus increases blood pressure and sympathetic outflow via vasopressin in hypertensive rats.Cardiovasc Res. 2013 Jun 1;98(3):344-51. doi: 10.1093/cvr/cvt031. Epub 2013 Feb 11. Cardiovasc Res. 2013. PMID: 23400761
-
Activation of afferent renal nerves modulates RVLM-projecting PVN neurons.Am J Physiol Heart Circ Physiol. 2015 May 1;308(9):H1103-11. doi: 10.1152/ajpheart.00862.2014. Epub 2015 Jan 30. Am J Physiol Heart Circ Physiol. 2015. PMID: 25637549 Free PMC article.
-
A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney.Exp Physiol. 2005 Mar;90(2):169-73. doi: 10.1113/expphysiol.2004.029041. Epub 2004 Dec 16. Exp Physiol. 2005. PMID: 15604110 Review.
References
-
- Abdelalim E. M., Masuda C., Bellier J. P., Saito A., Yamamoto S., Mori N., et al. (2008). Distribution of natriuretic peptide receptor-C immunoreactivity in the rat brainstem and its relationship to cholinergic and catecholaminergic neurons. Neuroscience 155 (1), 192–202. 10.1016/j.neuroscience.2008.05.020 - DOI - PubMed
-
- Anand I. S., Fisher L. D., Chiang Y. T., Latini R., Masson S., Maggioni A. P., et al. (2003). Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation 107 (9), 1278–1283. 10.1161/01.cir.0000054164.99881.00 - DOI - PubMed
-
- Anand-Srivastava M. B., Sairam M. R., Cantin M. (1990). Ring-deleted analogs of atrial natriuretic factor inhibit adenylate cyclase/cAMP system. Possible coupling of clearance atrial natriuretic factor receptors to adenylate cyclase/cAMP signal transduction system. J. Biol. Chem. 265 (15), 8566–8572. 10.1016/s0021-9258(19)38925-2 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

