A Methodological Review of Drug-Related Toxicological Studies in Saudi Arabia
- PMID: 37082486
- PMCID: PMC10112933
- DOI: 10.7759/cureus.36369
A Methodological Review of Drug-Related Toxicological Studies in Saudi Arabia
Abstract
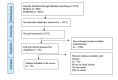
This study aimed to conduct a methodological review of drug-related toxicological studies in Saudi Arabia. A systematic review and a methodological analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Medline and Embase were searched for all types of studies reporting toxicological studies in the English language published until January 10, 2022. The search was conducted using both keywords and Medical Subject Headings (MeSH) terms. The methodological analysis of included studies was assessed using the Newcastle-Ottawa Scale. A total of 3,750 studies were extracted and screened. Of these, 30 observational studies (seven cohort studies and 23 cross-sectional studies) met the inclusion criteria. The methodological scores ranged from five to seven out of 10 possible points. Twelve studies had high quality, and 18 studies had moderate quality. Eight studies focused on adverse drug reactions, eight explored poisoning, four explored drug-related hospitalizations, nine explored drug-induced toxicity, and one explored drug overdose. This research project revealed that most of the drug-related toxicological studies conducted in Saudi Arabia were observational studies of moderate quality. Future studies should focus on the quality of the design and reporting.
Keywords: drug; overdose; substance; toxic; toxicology.
Copyright © 2023, Alwafi et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Reporting Quality of Cost-Effectiveness Analyses Conducted in Saudi Arabia: A Systematic Review.Value Health Reg Issues. 2021 Sep;25:99-103. doi: 10.1016/j.vhri.2020.12.012. Epub 2021 Apr 10. Value Health Reg Issues. 2021. PMID: 33848894
-
Association between pacifier use and breast-feeding, sudden infant death syndrome, infection and dental malocclusion.JBI Libr Syst Rev. 2005;3(6):1-33. doi: 10.11124/01938924-200503060-00001. JBI Libr Syst Rev. 2005. PMID: 27819973
-
Dental fluorosis prevalence in Saudi Arabia.Saudi Dent J. 2021 Nov;33(7):404-412. doi: 10.1016/j.sdentj.2021.03.007. Epub 2021 Mar 27. Saudi Dent J. 2021. PMID: 34803280 Free PMC article. Review.
-
The Etiology and Epidemiological Features of Acute Pancreatitis in Saudi Arabia: A Systematic Review.Cureus. 2023 Oct 5;15(10):e46511. doi: 10.7759/cureus.46511. eCollection 2023 Oct. Cureus. 2023. PMID: 37927657 Free PMC article. Review.
Cited by
-
Substance misuse disorder in Saudi Arabia: A comprehensive examination of current demographic patterns, trends, and intervention requirements.Saudi Pharm J. 2024 Oct;32(10):102163. doi: 10.1016/j.jsps.2024.102163. Epub 2024 Aug 16. Saudi Pharm J. 2024. PMID: 39262681 Free PMC article.
-
Parents' Knowledge, Attitude, and Practices toward Methamphetamine Abuse among Youth and its Risk Factors in Saudi Arabia.J Pharm Bioallied Sci. 2024 Feb;16(Suppl 1):S753-S756. doi: 10.4103/jpbs.jpbs_996_23. Epub 2024 Jan 5. J Pharm Bioallied Sci. 2024. PMID: 38595546 Free PMC article.
References
-
- Baud FJ, Houzé P. An Introduction to Interdisciplinary Toxicology: From Molecules to Man. Amsterdam, Netherlands: Elsevier; 2020. Introduction to clinical toxicology; pp. 413–428.
-
- Barile FA. Boca Raton, Florida: CRC Press; 2003. Clinical Toxicology: Principles and Mechanisms.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous