African American Prostate Cancer Displays Quantitatively Distinct Vitamin D Receptor Cistrome-transcriptome Relationships Regulated by BAZ1A
- PMID: 37082578
- PMCID: PMC10112383
- DOI: 10.1158/2767-9764.CRC-22-0389
African American Prostate Cancer Displays Quantitatively Distinct Vitamin D Receptor Cistrome-transcriptome Relationships Regulated by BAZ1A
Abstract
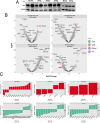
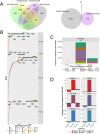
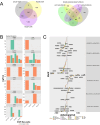
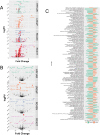
African American (AA) prostate cancer associates with vitamin D3 deficiency, but vitamin D receptor (VDR) genomic actions have not been investigated in this context. We undertook VDR proteogenomic analyses in European American (EA) and AA prostate cell lines and four clinical cohorts. Rapid immunoprecipitation mass spectrometry of endogenous protein (RIME) analyses revealed that nonmalignant AA RC43N prostate cells displayed the greatest dynamic protein content in the VDR complex. Likewise, in AA cells, Assay for Transposase-Accessible Chromatin using sequencing established greater 1α,25(OH)2D3-regulated chromatin accessibility, chromatin immunoprecipitation sequencing revealed significant enhancer-enriched VDR cistrome, and RNA sequencing identified the largest 1α,25(OH)2D3-dependent transcriptome. These VDR functions were significantly corrupted in the isogenic AA RC43T prostate cancer cells, and significantly distinct from EA cell models. We identified reduced expression of the chromatin remodeler, BAZ1A, in three AA prostate cancer cohorts as well as RC43T compared with RC43N. Restored BAZ1A expression significantly increased 1α,25(OH)2D3-regulated VDR-dependent gene expression in RC43T, but not HPr1AR or LNCaP cells. The clinical impact of VDR cistrome-transcriptome relationships were tested in three different clinical prostate cancer cohorts. Strikingly, only in AA patients with prostate cancer, the genes bound by VDR and/or associated with 1α,25(OH)2D3-dependent open chromatin (i) predicted progression from high-grade prostatic intraepithelial neoplasia to prostate cancer; (ii) responded to vitamin D3 supplementation in prostate cancer tumors; (iii) differentially responded to 25(OH)D3 serum levels. Finally, partial correlation analyses established that BAZ1A and components of the VDR complex identified by RIME significantly strengthened the correlation between VDR and target genes in AA prostate cancer only. Therefore, VDR transcriptional control is most potent in AA prostate cells and distorted through a BAZ1A-dependent control of VDR function.
Significance: Our study identified that genomic ancestry drives the VDR complex composition, genomic distribution, and transcriptional function, and is disrupted by BAZ1A and illustrates a novel driver for AA prostate cancer.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures


References
-
- Patel HD, Doshi CP, Koehne EL, Hart S, Van Kuiken M, Quek ML, et al. African American men have increased risk of prostate cancer detection despite similar rates of anterior prostatic lesions and PI-RADS grade on multiparametric magnetic resonance imaging. Urology 2022;163:132–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

