Subcellular localization of X-linked inhibitor of apoptosis protein (XIAP) in cancer: Does that matter?
- PMID: 37082602
- PMCID: PMC10074912
- DOI: 10.1016/j.bbadva.2022.100050
Subcellular localization of X-linked inhibitor of apoptosis protein (XIAP) in cancer: Does that matter?
Abstract
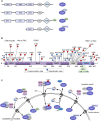
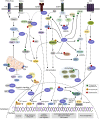
X-linked inhibitor of apoptosis protein (XIAP) finely tunes the balance between survival and death to control homeostasis. XIAP is found aberrantly expressed in cancer, which has been shown to promote resistance to therapy-induced apoptosis and confer poor outcome. Despite its predominant cytoplasmic localization in human tissues, growing evidence implicates the expression of XIAP in other subcellular compartments in sustaining cancer hallmarks. Herein, we review our current knowledge on the prognostic role of XIAP localization and discuss molecular mechanisms underlying differential biological functions played in each compartment. The comprehension of XIAP subcellular shuttling and functional dynamics might provide the rationale for future anticancer therapeutics.
Keywords: Cancer; Molecular functional mechanisms; Prognostic marker; Resistance to apoptosis; XIAP subcellular localization.
© 2022 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Expression of nuclear XIAP associates with cell growth and drug resistance and confers poor prognosis in breast cancer.Biochim Biophys Acta Mol Cell Res. 2020 Oct;1867(10):118761. doi: 10.1016/j.bbamcr.2020.118761. Epub 2020 May 30. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32485270
-
Upon drug-induced apoptosis in lymphoma cells X-linked inhibitor of apoptosis (XIAP) translocates from the cytosol to the nucleus.Leuk Lymphoma. 2004 Jul;45(7):1429-36. doi: 10.1080/1042819042000198858. Leuk Lymphoma. 2004. PMID: 15359644
-
X-linked inhibitor of apoptosis positive nuclear labeling: a new independent prognostic biomarker of breast invasive ductal carcinoma.Diagn Pathol. 2011 Jun 7;6:49. doi: 10.1186/1746-1596-6-49. Diagn Pathol. 2011. PMID: 21645409 Free PMC article.
-
IAPs as a target for anticancer therapy.Curr Cancer Drug Targets. 2007 Dec;7(8):785-94. doi: 10.2174/156800907783220471. Curr Cancer Drug Targets. 2007. PMID: 18220537 Review.
-
XIAP's Profile in Human Cancer.Biomolecules. 2020 Oct 29;10(11):1493. doi: 10.3390/biom10111493. Biomolecules. 2020. PMID: 33138314 Free PMC article. Review.
Cited by
-
SMAD7 Sustains XIAP Expression and Migration of Colorectal Carcinoma Cells.Cancers (Basel). 2024 Jun 28;16(13):2370. doi: 10.3390/cancers16132370. Cancers (Basel). 2024. PMID: 39001432 Free PMC article.
References
-
- Tamm I., Wang Y., Sausville E., Scudiero D.A., Vigna N., Oltersdorf T., Reed J.C. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

