Neoadjuvant Pseudomonas aeruginosa mannose-sensitive hemagglutinin (PA-MSHA) and chemotherapy versus placebo plus chemotherapy in patients with HER2-negative breast cancer: a randomized, controlled, double-blind trial
- PMID: 37082658
- PMCID: PMC10113104
- DOI: 10.21037/atm-22-4093
Neoadjuvant Pseudomonas aeruginosa mannose-sensitive hemagglutinin (PA-MSHA) and chemotherapy versus placebo plus chemotherapy in patients with HER2-negative breast cancer: a randomized, controlled, double-blind trial
Abstract
Background: According to preclinical experiments, Pseudomonas aeruginosa mannose-sensitive hemagglutinin (PA-MSHA) exerts antiproliferative effects against breast cancer cells. It has been approved by the State Food and Drug Administration in China for complementary cancer treatment, and its safety has been confirmed in previous clinical trials. The present randomized, controlled, double-blind clinical trial was conducted to investigate the efficacy and safety of neoadjuvant PA-MSHA and placebo with chemotherapy in patients with human epidermal growth factor receptor 2 (HER2)-negative breast cancer.
Methods: Eligible patients aged 18 years or older with previously untreated HER2-negative stage II-III breast cancer were enrolled and randomly assigned at a 1:1 ratio to receive neoadjuvant chemotherapy with PA-MSHA or a placebo. The Response Evaluation Criteria in Solid Tumors (RECIST) was used to assess clinical response every 2 cycles. The primary endpoint was the objective response rate (ORR) based on the clinical response following neoadjuvant chemotherapy.
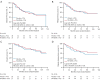
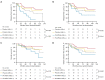
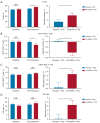
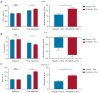
Results: A total of 75 patients were randomly assigned to either the PA-MSHA group (37 patients) or the control group (38 patients). The ORR was found to be significantly higher in the PA-MSHA group compared with the control group [86.5% versus 60.5%; rate difference 26.0; 95% confidence interval (CI): 5.9-43.5%; P=0.011]. The pathological complete response (pCR) and survival outcomes did not differ significantly between the 2 groups. Patients with immune-related adverse events (irAEs) appeared to benefit from the PA-MSHA treatment, with greater disease-free, relapse-free, and overall survival. The application of PA-MSHA to neoadjuvant chemotherapy did not increase the incidence of severe adverse events. Moreover, the addition of PA-MSHA increased serum interferon-γ levels and the percentage of peripheral blood T cells, CD8+/CD4+ T cells, CD8+CD28+ T cells, and natural killer (NK) cells, and decreased serum interleukin 4 levels.
Conclusions: The addition of PA-MSHA to neoadjuvant chemotherapy is an effective alternative regimen for HER2-negative breast cancer. Patients with irAEs caused by PA-MSHA may obtain more benefits from this treatment.
Trial registration: Chinese Clinical Trial Registry ChiCTR-TRC-10000794.
Keywords: HER2-negative breast cancer; Pseudomonas aeruginosa mannose-sensitive hemagglutinin (PA-MSHA); clinical trial; efficacy; neoadjuvant chemotherapy.
2023 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4093/coif). The authors have no conflicts of interest to declare.
Figures
Comment in
-
Neoadjuvant immunotherapy in early-stage triple negative breast cancer.Ann Transl Med. 2023 Aug 30;11(10):338. doi: 10.21037/atm-23-1550. Epub 2023 Jun 27. Ann Transl Med. 2023. PMID: 37675326 Free PMC article. No abstract available.
References
-
- Yu KD, Wu SY, Liu GY, et al. Concurrent neoadjuvant chemotherapy and estrogen deprivation in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (CBCSG-036): A randomized, controlled, multicenter trial. Cancer 2019;125:2185-93. 10.1002/cncr.32057 - DOI - PubMed
-
- Samiei S, Simons JM, Engelen SME, et al. Axillary Pathologic Complete Response After Neoadjuvant Systemic Therapy by Breast Cancer Subtype in Patients With Initially Clinically Node-Positive Disease: A Systematic Review and Meta-analysis. JAMA Surg 2021;156:e210891. 10.1001/jamasurg.2021.0891 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
