The efficacy and safety of nirsevimab for the prevention of RSV infection among infants: A systematic review and meta-analysis
- PMID: 37082704
- PMCID: PMC10110918
- DOI: 10.3389/fped.2023.1132740
The efficacy and safety of nirsevimab for the prevention of RSV infection among infants: A systematic review and meta-analysis
Abstract
Background: Respiratory syncytial virus (RSV) is a leading cause of morbidity and mortality among infants with a global incidence of 9.5% and a mortality rate of 2.2%. The management of RSV infection is mainly supportive and, aside from emerging monoclonal antibodies, there has been no benefit of most preventive measures. Recent evidence suggests the potential of nirsevimab in preventing RSV infection.
Objective: This study aims to determine the efficacy and safety of nirsevimab in preventing RSV infection among infants using a review of relevant clinical trials.
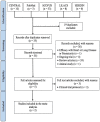
Methods: We performed a random-effects meta-analysis among infants comparing nirsevimab injection vs. placebo. MEDLINE, CENTRAL, Scopus, and ClinicalTrials.gov were searched for relevant trials from inception to June 2022. The selected studies were assessed for risk of bias using the Revised Cochrane Risk-of-Bias (RoB2) tool and for quality of evidence using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach.
Results: Two studies were included. Data analysis showed that among infants, nirsevimab given before the RSV season significantly reduced the risk of medically attended RSV-related infection (RR: 0.26; 95% CI: 0.18-0.38) and the risk of hospitalization due to RSV infection (RR: 0.24; 95% CI: 0.13-0.47). There was no difference in terms of adverse events leading to death (RR: 0.78, 95% CI: 0.20-2.98) and adverse events of special interest (RR: 0.92, 95% CI: 0.25-3.38).
Conclusions: The use of nirsevimab to prevent RSV infections and hospitalization shows its promising potential, but studies on its cost-effectiveness are lacking. We recommend that further studies be done to look into the applicability and cost-effectiveness of nirsevimab.
Keywords: infants; lower respiratory tract infection; nirsevimab; prevention; respiratory syncytial virus.
© 2023 Turalde-Mapili, Mapili, Turalde and Pagcatipunan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399(10340):2047–64. 10.1016/S0140-6736(22)00478-0 - DOI - PMC - PubMed
-
- Ventre K, Randolph A. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. In: Ventre K, editors. Cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd; (2007):1–19. Available at: https://doi.wiley.com/10.1002/14651858.CD000181.pub3. - DOI - PubMed
-
- Fuller HL, Del Mar C. Immunoglobulin treatment for respiratory syncytial virus infection. In: Fuller HL, editors. Cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd; (2006). p. 1–13. Available at: https://doi.wiley.com/10.1002/14651858.CD004883.pub2. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources