Clinical features and "early" corticosteroid treatment outcome of pediatric mycoplasma pneumoniae pneumonia
- PMID: 37082710
- PMCID: PMC10110948
- DOI: 10.3389/fcimb.2023.1135228
Clinical features and "early" corticosteroid treatment outcome of pediatric mycoplasma pneumoniae pneumonia
Abstract
Background: Many children with mycoplasma pneumoniae (MP) pneumonia (MPP) developed sequelae such as bronchiolitis/bronchitis obliterans (BO). Early corticosteroid therapy might prevent disease progression. This study aimed to use "early" corticosteroid and observe the treatment outcome in patients with MPP.
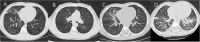
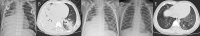
Methods: Patients who had pulmonary infiltrations on chest imaging within 5 days of the disease course and were suspected of having MP infection on admission were enrolled. Among them, patients whose disease course was within 10 days on admission were ultimately enrolled. We analyzed their data including the clinical features, the starting time and dose of corticosteroid therapy, and the treatment outcome. According to chest imaging, we divided patients into two groups (Group A: bronchiolitis-associated lesions or ground-glass opacities; Group B: pulmonary segmental/lobar consolidation).
Results: A total of 210 patients with confirmed MPP were ultimately enrolled. There were 59 patients in Group A and 151 patients in Group B. Patients in Group A were more prone to have allergy histories, hypoxemia, wheezing sound, and wet rales on auscultation than those in Group B. Corticosteroid treatment was initiated between 5 and 10 days of disease onset in all patients and 6-7 days in most patients. Methylprednisolone was prescribed in all patients within 10 days of disease onset, and the highest prescribed dose was at least 2 mg/kg/day. In Group A, methylprednisolone >2 mg/kg/day was prescribed in 22 patients, and among them, 8 patients with diffuse bronchiolitis-associated lesions received high-dose methylprednisolone therapy. After 3 months, lung CT revealed slightly segmental ground-glass opacity in three patients. In Group B, methylprednisolone >2 mg/kg/day was prescribed in 76 patients, and among them, 20 patients with pulmonary lobar consolidation received high-dose methylprednisolone therapy. After 3 months, chest imaging revealed incomplete absorption of pulmonary lesions in seven patients. Among them, five patients with consolidation in more than one pulmonary lobe ultimately had slight BO.
Conclusion: In hospitalized patients with MPP, particularly severe MPP, the ideal starting time of corticosteroid treatment might be 5-10 days, preferably 6-7 days, after disease onset. The initial dosage of corticosteroid therapy should be decided according to the severity of the disease. MPP patients with diffuse bronchiolitis-associated lesions/whole lobar consolidation on imaging might require high-dose corticosteroid therapy.
Keywords: Mycoplasma pneumoniae; children; corticosteroid; outcome; pneumonia.
Copyright © 2023 Liu, He, Zhang, Zhao, Liu, Wang and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

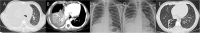

References
-
- Guo Z., Liu L., Gong J., Han N., He L., Wang W., et al. . (2022). Molecular features and antimicrobial susceptibility of mycoplasma pneumoniae isolates from paediatric inpatients in weihai, China: Characteristics of m. pneumoniae in weihai. J. Glob Antimicrob. Resist. 28, 180–184. doi: 10.1016/J.Jgar.2022.01.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

