Histone H3 activates caspase-1 and promotes proliferation and metastasis in hepatocellular carcinoma
- PMID: 37082731
- PMCID: PMC10110467
- DOI: 10.7150/ijms.76580
Histone H3 activates caspase-1 and promotes proliferation and metastasis in hepatocellular carcinoma
Abstract
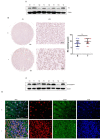
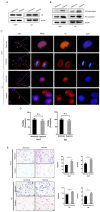
Background: As a component of nucleosomes, histone H3 plays an important role in chromosome structure and gene expression. Current studies have mostly focused on the role of histones in epigenetics, but in addition to this, the role of histones themselves in tumor development and microenvironment have been less explored. Methods: Western blot and immunofluorescence were carried out to detect the content and localization of histone H3 in hepatocellular carcinoma. The changes of histone H3 were observed in hypoxia treatment cells, the specific action mechanism of histone H3 was studied by CoIP and other methods. Cell Counting Kit-8 assay, plate cloning assay and transwell assay were used to exam the effect of histone H3 on cell proliferation and metastasis, which were verified by subcutaneous tumors in mice and lung metastasis by tail vein injection in mice. Results: We found that histone H3 was overexpressed in hepatocellular carcinoma tumor tissues compared to adjacent non-tumor tissues, and there was concomitant translocation of histone H3 from the nucleus to the cytoplasm. We found that hypoxia could contribute to this phenomenon of histone H3 translocation from the nucleus to the cytoplasm in hepatocellular carcinoma cells and increased binding levels to TLR9. At the same time, hypoxia induced downstream activation of TLR9 and caspase-1, as well as cleavage and release of the pro-inflammatory cytokines IL-1β and IL-18. We further demonstrated that histone H3 could also promote proliferation and metastasis of hepatocellular carcinoma through TLR9 activation of NLRP3 inflammasome. In addition, overexpression of histone H3 was also confirmed to promote hepatocellular carcinoma proliferation and metastasis in mouse models of hepatocellular carcinoma growth assay and lung metastasis. Conclusions: In hypoxic hepatocellular carcinoma cells, histone H3 can translocate to the cytoplasm and activate caspase-1 via TLR9, thereby producing pro-inflammatory cytokines that promote tumor proliferation and metastasis.
Keywords: Hepatocellular carcinoma; Histone H3; Hypoxia; NLRP3; TLR9.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9.J Hepatol. 2015 Jul;63(1):114-21. doi: 10.1016/j.jhep.2015.02.009. Epub 2015 Feb 12. J Hepatol. 2015. PMID: 25681553 Free PMC article.
-
MiR-887 Promotes the Progression of Hepatocellular Carcinoma via Targeting VHL.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820940425. doi: 10.1177/1533033820940425. Technol Cancer Res Treat. 2020. PMID: 32912113 Free PMC article.
-
High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases.Hepatology. 2012 Jun;55(6):1863-75. doi: 10.1002/hep.25572. Hepatology. 2012. PMID: 22234969 Free PMC article.
-
Cytokeratin 18-associated Histone 3 Modulation in Hepatocellular Carcinoma: A Mini Review.Cancer Genomics Proteomics. 2017 Jul-Aug;14(4):219-223. doi: 10.21873/cgp.20033. Cancer Genomics Proteomics. 2017. PMID: 28647696 Free PMC article. Review.
-
The cancer driver genes IDH1/2, JARID1C/ KDM5C, and UTX/ KDM6A: crosstalk between histone demethylation and hypoxic reprogramming in cancer metabolism.Exp Mol Med. 2019 Jun 20;51(6):1-17. doi: 10.1038/s12276-019-0230-6. Exp Mol Med. 2019. PMID: 31221981 Free PMC article. Review.
Cited by
-
Anti-DAMP therapies for acute inflammation.Front Immunol. 2025 May 8;16:1579954. doi: 10.3389/fimmu.2025.1579954. eCollection 2025. Front Immunol. 2025. PMID: 40406124 Free PMC article. Review.
-
Toll-Like Receptors in the Immunotherapy Era: Dual-Edged Swords of Tumor Immunity and Clinical Translation.MedComm (2020). 2025 Jul 27;6(8):e70308. doi: 10.1002/mco2.70308. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40727252 Free PMC article. Review.
-
HMGB1 promotes mitochondrial transfer between hepatocellular carcinoma cells through RHOT1 and RAC1 under hypoxia.Cell Death Dis. 2024 Feb 20;15(2):155. doi: 10.1038/s41419-024-06536-6. Cell Death Dis. 2024. PMID: 38378644 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021. - PubMed
-
- Li J, Liao T, Liu H, Yuan H, Ouyang T, Wang J, Chai S, Li J, Chen J, Li X, Zhao H, Xiong N. Hypoxic Glioma Stem Cell-Derived Exosomes Containing Linc01060 Promote Progression of Glioma by Regulating the MZF1/c-Myc/HIF1α Axis. Cancer Res. 2021;81:114–128. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

