Discovery of a High-Affinity Fluoromethyl Analog of [11C]5-Cyano- N-(4-(4-methylpiperazin-1-yl)-2-(piperidin-1-yl)phenyl)furan-2-carboxamide ([11C]CPPC) and Their Comparison in Mouse and Monkey as Colony-Stimulating Factor 1 Receptor Positron Emission Tomography Radioligands
- PMID: 37082755
- PMCID: PMC10111626
- DOI: 10.1021/acsptsci.3c00003
Discovery of a High-Affinity Fluoromethyl Analog of [11C]5-Cyano- N-(4-(4-methylpiperazin-1-yl)-2-(piperidin-1-yl)phenyl)furan-2-carboxamide ([11C]CPPC) and Their Comparison in Mouse and Monkey as Colony-Stimulating Factor 1 Receptor Positron Emission Tomography Radioligands
Abstract
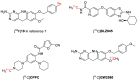
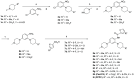
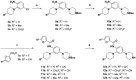
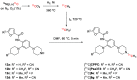
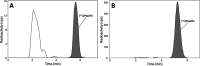
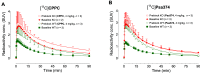
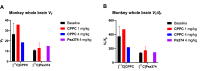
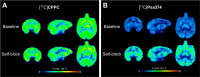
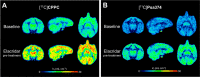
[11C]CPPC has been advocated as a radioligand for colony-stimulating factor 1 receptor (CSF1R) with the potential for imaging neuroinflammation in human subjects with positron emission tomography (PET). This study sought to prepare fluoro analogs of CPPC with higher affinity to provide the potential for labeling with longer-lived fluorine-18 (t 1/2 = 109.8 min) and for delivery of higher CSF1R-specific PET signal in vivo. Seven fluorine-containing analogs of CPPC were prepared and four were found to have high inhibitory potency (IC50 in low to sub-nM range) and selectivity at CSF1R comparable with CPPC itself. One of these, a 4-fluoromethyl analog (Psa374), was investigated more deeply by labeling with carbon-11 (t 1/2 = 20.4 min) for PET studies in mouse and monkey. [11C]Psa374 showed high peak uptake in monkey brain but not in mouse brain. Pharmacological challenges revealed no CSF1R-specific binding in either species at baseline. [11C]CPPC also failed to show specific binding at baseline. Moreover, both [11C]Psa374 and [11C]CPPC showed brain efflux transporter substrate behavior in both species in vivo, although Psa374 did not show liability toward human efflux transporters in vitro. Further development of [11C]Psa374 in non-human primate models of neuroinflammation with demonstration of CSF1R-specific binding would be required to warrant the fluorine-18 labeling of Psa374 with a view to possible application in human subjects.
Not subject to U.S. Copyright. Published 2023 by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Ginhoux F.; Greter M.; Leboeuf M.; Nandi S.; See P.; Gokhan S.; Mehler M. F.; Conway S. J.; Ng L. G.; Stanley E. R.; Samokhvalov I. M.; Merad M. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 2010, 330 (6005), 841–845. 10.1126/science.1194637. - DOI - PMC - PubMed
-
- Zhang Y.; Chen K.; Sloan S. A.; Bennett M. L.; Scholze A. R.; O’Keeffe S.; Phatnani H. P.; Guarnieri P.; Caneda C.; Ruderisch N.; Deng S.; Liddelow S. A.; Zhang C.; Daneman R.; Maniatis T.; Barres B. A.; Wu J. Q. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014, 34 (36), 11929–11947. 10.1523/JNEUROSCI.1860-14.2014. - DOI - PMC - PubMed
-
- Chen Z.; Haider A.; Chen J.; Xiao Z.; Gobbi L.; Honer M.; Grether U.; Arnold S. E.; Josephson L.; Liang S. H. The Repertoire of Small-Molecule PET Probes for Neuroinflammation Imaging: Challenges and Opportunities beyond TSPO. J. Med. Chem. 2021, 64 (24), 17656–17689. 10.1021/acs.jmedchem.1c01571. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
