Neurochemical alterations of intrinsic cardiac ganglionated nerve plexus caused by arterial hypertension developed during ageing in spontaneously hypertensive and Wistar Kyoto rats
- PMID: 37083051
- PMCID: PMC10485580
- DOI: 10.1111/joa.13877
Neurochemical alterations of intrinsic cardiac ganglionated nerve plexus caused by arterial hypertension developed during ageing in spontaneously hypertensive and Wistar Kyoto rats
Abstract
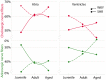
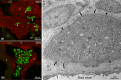
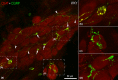
The acknowledged hypothesis of the cause of arterial hypertension is the emerging disbalance in sympathetic and parasympathetic regulations of the cardiovascular system. This disbalance manifests in a disorder of sustainability of endogenous autonomic and sensory neural substances including calcitonin gene-related peptide (CGRP). This study aimed to examine neurochemical alterations of intrinsic cardiac ganglionated nerve plexus (GP) triggered by arterial hypertension during ageing in spontaneously hypertensive rats of juvenile (prehypertensive, 8-9 weeks), adult (early hypertensive, 12-18 weeks) and elderly (persistent hypertensive, 46-60 weeks) age in comparison with the age-matched Wistar-Kyoto rats as controls. Parasympathetic, sympathetic and sensory neural structures of GP were analysed and evaluated morphometrically in tissue sections and whole-mount cardiac preparations. Both the elevated blood pressure and the evident ultrasonic signs of heart failure were identified for spontaneously hypertensive rats and in part for the aged control rats. The amount of adrenergic and immunoreactive to CGRP neural structures was increased in the adult group of spontaneously hypertensive rats along with the significant alterations that occurred during ageing. In conclusion, the revealed chemical alterations of GP support the hypothesis about the possible disbalance of efferent and afferent heart innervation and may be considered as the basis for the emergence and progression of arterial hypertension and perhaps even as a consequence of hypertension in the aged spontaneously hypertensive rats. The determined anatomical changes in the ageing Wistar-Kyoto rats suggest this breed being as inappropriate for its use as control animals for hypertension studies in older animal age.
Keywords: CGRP; ChAT; SIF cells; TH; heart ganglia, nerves and plexus; hypertension; immunohistochemistry.
© 2023 Anatomical Society.
Figures
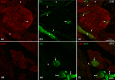
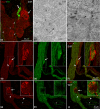
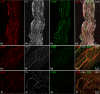
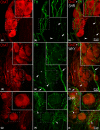

Similar articles
-
Comparative analysis of intracardiac neural structures in the aged rats with essential hypertension.Anat Rec (Hoboken). 2023 Sep;306(9):2313-2332. doi: 10.1002/ar.25109. Epub 2022 Nov 7. Anat Rec (Hoboken). 2023. PMID: 36342958
-
Early structural alterations of intrinsic cardiac ganglionated plexus in spontaneously hypertensive rats.Histol Histopathol. 2022 Oct;37(10):955-970. doi: 10.14670/HH-18-453. Epub 2022 Mar 31. Histol Histopathol. 2022. PMID: 35356999
-
Age-related decrease of calcitonin gene-related peptide-containing vasodilator innervation in the mesenteric resistance vessel of the spontaneously hypertensive rat.Circ Res. 1990 Sep;67(3):733-43. doi: 10.1161/01.res.67.3.733. Circ Res. 1990. PMID: 2397578
-
[Role of angiontensin receptors in remodeling perivascular nerves].Yakugaku Zasshi. 2010 Nov;130(11):1421-5. doi: 10.1248/yakushi.130.1421. Yakugaku Zasshi. 2010. PMID: 21048398 Review. Japanese.
-
Changes in arterial smooth muscle contractility, contractile proteins, and arterial wall structure in spontaneous hypertension.Proc Soc Exp Biol Med. 1994 Nov;207(2):148-74. doi: 10.3181/00379727-207-43802. Proc Soc Exp Biol Med. 1994. PMID: 7938046 Review.
References
-
- Adams, M.A. , Bobik, A. & Korner, P.I. (1989) Differential development of vascular and cardiac hypertrophy in genetic hypertension. Relation to sympathetic function. Hypertension (Dallas, Tex: 1979), 14(2), 191–202. - PubMed
-
- Aksu, T. , Gopinathannair, R. , Gupta, D. & Pauza, D.H. (2021) Intrinsic cardiac autonomic nervous system: what do clinical electrophysiologists need to know about the “heart brain”? Journal of Cardiovascular Electrophysiology, 32(6), 1737–1747. - PubMed
-
- Ashton, J.L. , Argent, L. , Smith, J.E.G. , Jin, S. , Sands, G.B. , Smaill, B.H. et al. (2020) Evidence of structural and functional plasticity occurring within the intracardiac nervous system of spontaneously hypertensive rats. American Journal of Physiology. Heart and Circulatory Physiology, 318(6), H1387–H1400. - PubMed
-
- Atanasova, D.Y. , Iliev, M.E. & Lazarov, N.E. (2011) Morphology of the rat carotid body. Biomedical Reviews, 22, 41–55.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

