Paclitaxel induces pyroptosis by inhibiting the volume‑sensitive chloride channel leucine‑rich repeat‑containing 8a in ovarian cancer cells
- PMID: 37083067
- PMCID: PMC10170489
- DOI: 10.3892/or.2023.8552
Paclitaxel induces pyroptosis by inhibiting the volume‑sensitive chloride channel leucine‑rich repeat‑containing 8a in ovarian cancer cells
Abstract
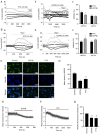
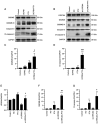
Pyroptosis is a newly identified form of cell death, morphologically characterized by excessive cell swelling. In the present study, paclitaxel (PTX) combined with platinum were used as first‑line chemotherapy, against ovarian cancer cells by inducing multiple types of cell death. However, it remains unclear whether PTX can induce pyroptosis in ovarian cancer cells. It was recently reported that PTX inhibited chloride channels, an inhibition known to cause cell swelling. In the present study, it was first verified that pyroptosis‑like cell death, as well as cleaved‑caspase‑3 and cleaved‑gasdermin E (GSDME) were induced by PTX in A2780 ovarian cancer cells. PTX inhibited the background‑ and hypotonicity‑activated chloride currents, promoted intracellular chloride ion accumulation, those manifestations are similar to those of the classic volume‑regulatory anion channel (VRAC) blocker, 4‑(2‑butyl‑6,7‑dichloro‑2‑cy-clopentyl‑indan‑1‑on5‑yl) oxobutyric acid (DCPIB). Of note, both DCPIB and the downregulation of VRAC constituent protein leucine‑rich repeat‑containing 8a themselves could not induce persisted cell swelling and pyroptosis‑like phenotypes. However, they could enhance the effects of PTX in inducing pyroptosis‑like phenotypes, such as marked cell swelling, cell membrane rupture and excessive activation of caspase‑3 and GSDME N‑terminal fragment, which ultimately caused marked pyroptosis in A2780 cells. These findings revealed a potential mechanism of PTX and offered new insights into the effects of a synergistical combination of PTX and VRACs blockers in ovarian cancer chemotherapy.
Keywords: chloride channel; ovarian cancer; paclitaxel; pyroptosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

