The Treat-to-Target Project in Atopic Dermatitis: One Year On
- PMID: 37083095
- PMCID: PMC10134065
- DOI: 10.2340/actadv.v103.5382
The Treat-to-Target Project in Atopic Dermatitis: One Year On
Abstract
Atopic dermatitis is a chronic skin condition for which a range of systemic treatments have recently been approved. A treat-to-target strategy has been developed previously alongside an algorithm to guide the management of patients with atopic dermatitis. Here, we review the strategy and algorithm in the context of the evolving therapeutic landscape, and identify areas for further refinement and development.
Conflict of interest statement
MdB-W is co-principal investigator of the Dutch BioDay Registry and serves as a principal investigator in a number of multi-center clinical trials in atopic dermatitis sponsored by a wide range of pharmaceutical companies; has attended advisory boards and educational events sponsored by Sanofi, Regeneron, AbbVie and Galderma; has received institutional research grants from Sanofi; and has performed consultancy roles with Sanofi, Regeneron, LEO Pharma, Eli Lilly, AbbVie, Pfizer, Galderma and UCB, outside the submitted work. TB has received speaker and consultancy fees and has received institutional research grants from Alk-Abelló, Amgen, Almirall, Celgene, Galderma, LEO Pharma, Eli Lilly, Mylan, Novartis, Phadia-Thermo-Fisher, Regeneron, Sanofi-Genzyme, and Viatris outside the submitted work. RB is an employee and shareholder of Innovaderm Research; has received speaker and consultancy fees, and has received institutional research grants from AbbVie, Arcutis, Arena Pharma, Asana BioSciences, Bellus Health, Bluefin Biomedicine, BioMimetix, Boehringer-Ingelheim, Boston, Brickell, CARA Therapeutic, Clexio, Dermavant, Eli Lilly, EMD Serono, Evidera, Galderma, GlaxoSmithKline, Incyte, Inmagene Bio, Janssen, Kiniksa, Kyowa Kirin, LEO Pharma, Novan, Novartis, Pfizer, Ralexar, RAPT Therapeutic, Regeneron, Respivant, Sanofi, Sienna, Target RWE and Vyne Therapeutics. MD has received speaker fees, served as a consultant, and/or has received institutional research grants from AbbVie, Eli Lilly, Aslan Pharmaceuticals, Arena Pharmaceuticals, Incyte, LEO Pharma, Pfizer, La Roche Posay, Pierre Fabre, Regeneron Pharmaceuticals, Inc., and Sanofi, outside the submitted work. PF has attended advisory boards for AbbVie, Amgen, Aslan, Boehringer-Ingelheim, Bristol Myers Squibb, Celgene, Galderma, GSK, Janssen, LEO Pharma, Eli Lilly, Mayne Pharma, Merck, Novartis, Pfizer, Sanofi, Sun Pharma, UCB Pharma, and Valeant; has served as a consultant to Aslan, Bristol Myers Squibb, Galderma, GenesisCare, Hexima, Janssen, LEO Pharma, Eli Lilly, Mayne Pharma, MedImmune, Novartis, Pfizer, Roche, and UCB Pharma; has acted as an investigator in clinical trials for AbbVie, Akaal, Amgen, Argenx, Aslan, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharma, UCB Pharma, Valeant, Astra Zeneca, BMS, Botanix, Celtaxsys, CSL, Cutanea, Dermira, Evelo, Galderma, Genentech, GSK, Hexima, Kymab, Regeneron Pharmaceuticals Inc., Roche, Teva, and Sanofi; has received institutional research grants from AbbVie, Amgen, Celgene, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharma, Galderma, and Sanofi; has received speaker fees or other honoraria from AbbVie, Amgen, Celgene, Janssen, LEO Pharma, Eli Lilly, Merck, Novartis, Pfizer, UCB Pharma, Valeant, Galderma, GSK, Roche, and Sanofi; and has received travel expenses for attendance at educational meetings from AbbVie, Janssen, LEO Pharma, Eli Lilly, Merck, Novartis, Pfizer, Sun Pharma, Galderma, Roche, and Sanofi, outside the submitted work. GG has received personal fees from AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Fresenius Kabi, Galderma, Genzyme, LEO Pharma, Novartis, Pfizer, Regeneron, Samsung and Sanofi. JH has attended advisory boards for Sanofi Genzyme, LEO Pharma and UCB; has acted as an investigator in clinical studies sponsored by Diasorin and Eli Lilly; and has received speaker fees from UCB, Janssen, Sanofi-Aventis, Eli Lilly, Novartis and AbbVie. C-HH has received honoraria as a speaker/consultant for AbbVie, Amgen, Bausch Health, Celgene, Eli Lilly, Galderma, Glaxo-Smith-Kline, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, and UCB; and has received grants as an investigator from AbbVie, Amgen, Bausch Health, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Dermavant, Eli Lilly, Galderma, Glaxo-Smith-Kline, Incyte, Janssen, LEO Pharma, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, and UCB, outside the submitted work. NK has received honoraria as a speaker/consultant for AbbVie, Celgene Japan, Janssen Pharma, Kyowa Kirin, LEO Pharma, Eli Lilly Japan, Maruho, Mitsubishi Tanabe Pharma, Sanofi, Taiho Pharmaceutical, and Torii Pharmaceutical, and has received grants as an investigator from AbbVie, A2 Healthcare, Boehringer Ingelheim Japan, Eisai, Janssen Pharma, Kyowa Kirin, LEO Pharma, Eli Lilly Japan, Maruho, Sun Pharma, Taiho Pharmaceutical, and Torii Pharmaceutical. AEP has acted as an advisor, speaker, investigator or received educational support from: Sanofi, AbbVie, Eli Lilly, Pfizer, Galderma, Novartis, Almirall, La-Roche Posay, LEO, UCB, BMS, Amgen, Celgene, Novartis, and Janssen. SS has received honoraria as a speaker/consultant for Sanofi and has received institutional research grants as an investigator from Sanofi, outside of submitted work. JS reports institutional grants for investigator-initiated research from the German GBA, the BMG, BMBF, EU, Federal State of Saxony, Novartis, Sanofi, ALK, and Pfizer. He also participated in advisory board meetings as a paid consultant for Sanofi, Eli Lilly, and ALK. JT is an advisor for AbbVie, Almirall, Arena Pharmaceuticals, Coloplast, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, a speaker for AbbVie, Almirall, Eli Lilly, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, and received research grants from Pfizer, Regeneron, and Sanofi-Genzyme. SW is the co-principal investigator of the German Atopic Eczema Registry (TREATgermany) and coordinator of the EU/IMI project BIOMAP (BIOMarkers in Atopic dermatitis and Psoriasis) projects and is an investigator in a number of clinical trials in atopic dermatitis and psoriasis sponsored by a wide range of pharmaceutical companies; has received institutional research grants from Sanofi, LEO Pharma and L’Oréal; has acted as a consultant for Sanofi, Regeneron, LEO Pharma, Eli Lilly, AbbVie, Pfizer, Incyte and Novartis; and has lectured at educational events sponsored by Sanofi, Regeneron, LEO Pharma, AbbVie and Galderma, outside of submitted work.
Figures
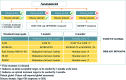
References
-
- Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. . Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682. - PubMed
-
- Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. . Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32: 850–878. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

