Carbon monoxide separation: past, present and future
- PMID: 37083229
- PMCID: PMC10243283
- DOI: 10.1039/d3cs00147d
Carbon monoxide separation: past, present and future
Abstract
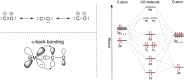
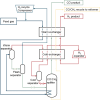
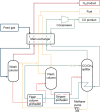
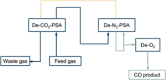
Large amounts of carbon monoxide are produced by industrial processes such as biomass gasification and steel manufacturing. The CO present in vent streams is often burnt, this produces a large amount of CO2, e.g., oxidation of CO from metallurgic flue gasses is solely responsible for 2.7% of manmade CO2 emissions. The separation of N2 from CO due to their very similar physical properties is very challenging, meaning that numerous energy-intensive steps are required for CO separation, making the CO separation from many process streams uneconomical in spite of CO being a valuable building block in the production of major chemicals through C1 chemistry and the production of linear hydrocarbons by the Fischer-Tropsch process. The development of suitable processes for the separation of carbon monoxide has both industrial and environmental significance. Especially since CO is a main product of electrocatalytic CO2 reduction, an emerging sustainable technology to enable carbon neutrality. This technology also requires an energy-efficient separation process. Therefore, there is a great need to develop energy efficient CO separation processes adequate for these different process streams. As such the urgency of separating carbon monoxide is gaining greater recognition, with research in the field becoming more and more crucial. This review details the principles on which CO separation is based and provides an overview of currently commercialised CO separation processes and their limitations. Adsorption is identified as a technology with the potential for CO separation with high selectivity and energy efficiency. We review the research efforts, mainly seen in the last decades, in developing new materials for CO separation via ad/bsorption and membrane technology. We have geared our review to both traditional CO sources and emerging CO sources, including CO production from CO2 conversion. To that end, a variety of emerging processes as potential CO2-to-CO technologies are discussed and, specifically, the need for CO capture after electrochemical CO2 reduction is highlighted, which is still underexposed in the available literature. Altogether, we aim to highlight the knowledge gaps that could guide future research to improve CO separation performance for industrial implementation.
Conflict of interest statement
There are no conflicts to declare.
Figures










References
-
- Wall D. Kepplinger W. Millner R. Steel Res. Int. 2011;82:926–933. doi: 10.1002/srin.201100030. - DOI
-
- Bierhals J., Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2001
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

