Tissue-resident memory T cell maintenance during antigen persistence requires both cognate antigen and interleukin-15
- PMID: 37083450
- PMCID: PMC10334460
- DOI: 10.1126/sciimmunol.add8454
Tissue-resident memory T cell maintenance during antigen persistence requires both cognate antigen and interleukin-15
Abstract
Our understanding of tissue-resident memory T (TRM) cell biology has been largely developed from acute infection models in which antigen is cleared and sterilizing immunity is achieved. Less is known about TRM cells in the context of chronic antigen persistence and inflammation. We investigated factors that underlie TRM maintenance in a kidney transplantation model in which TRM cells drive rejection. In contrast to acute infection, we found that TRM cells declined markedly in the absence of cognate antigen, antigen presentation, or antigen sensing by the T cells. Depletion of graft-infiltrating dendritic cells or interruption of antigen presentation after TRM cells were established was sufficient to disrupt TRM maintenance and reduce allograft pathology. Likewise, removal of IL-15 transpresentation or of the IL-15 receptor on T cells during TRM maintenance led to a decline in TRM cells, and IL-15 receptor blockade prevented chronic rejection. Therefore, antigen and IL-15 presented by dendritic cells play nonredundant key roles in CD8 TRM cell maintenance in settings of antigen persistence and inflammation. These findings provide insights that could lead to improved treatment of chronic transplant rejection and autoimmunity.
Conflict of interest statement
Figures

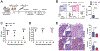
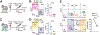

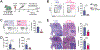


References
-
- Ibrahim S, Dawson DV, Sanfilippo F, Predominant infiltration of rejecting human renal allografts with T cells expressing CD8 and CD45RO. Transplantation 59, 724–728 (1995). - PubMed
-
- Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell LD, Clahsen-van Groningen MC, Demetris AJ, Dragun D, Duong van Huyen JP, Farris AB, Fogo AB, Gibson IW, Glotz D, Gueguen J, Kikic Z, Kozakowski N, Kraus E, Lefaucheur C, Liapis H, Mannon RB, Montgomery RA, Nankivell BJ, Nickeleit V, Nickerson P, Rabant M, Racusen L, Randhawa P, Robin B, Rosales IA, Sapir-Pichhadze R, Schinstock CA, Seron D, Singh HK, Smith RN, Stegall MD, Zeevi A, Solez K, Colvin RB, Mengel M, The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. American Journal of Transplantation 20, 2318–2331 (2020). - PMC - PubMed
-
- Rosenberg AS, Singer A, Cellular Basis of Skin Allograft Rejection: An In Vivo Model of Immune-Mediated Tissue Destruction. Annual review of immunology 10, 333–358 (1992). - PubMed
-
- Hancock WW, Gao W, Faia KL, Csizmadia V, Chemokines and their receptors in allograft rejection. Current opinion in immunology 12, 511–516 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

