Efficacy and safety of vesatolimod in chronic hepatitis B: A systematic review and meta-analysis
- PMID: 37083786
- PMCID: PMC10118312
- DOI: 10.1097/MD.0000000000033609
Efficacy and safety of vesatolimod in chronic hepatitis B: A systematic review and meta-analysis
Abstract
Background: Vesatolimod is a toll-like receptor (TLR) agonist that is thought to suppress chronic hepatitis B (HBV) infection. This systematic review aimed to assess the safety and efficacy of vesatolimod in treating chronic hepatitis B.
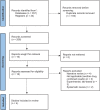
Methods: We included randomized clinical trials (RCTs) that assessed vesatolimod in patients with hepatitis B infection without hepatocellular carcinoma or liver transplantation and with reported levels of hepatitis B surface antigen (HBsAg) or liver transaminases post-intervention. We searched MEDLINE, SCOPUS, Springer, Google Scholar, ClinicalTrials.gov, and Cochrane Central Register of Clinical Trials for all related articles during May 2022. Two independent authors screened articles for inclusion, and discrepancies were resolved by consensus and a third reviewer. Two independent reviewers assessed studies included in this systematic review using the Critical Appraisal Skills Programme checklist for RCTs.
Results and conclusion: Only 4 were considered eligible from 391 articles identified through our search. All eligible studies did not report any clinically significant outcomes following the use of vesatolimod, as evidenced by the persistence of HBsAg. However, vesatolimod was associated with induction of interferon-stimulated genes (ISGs) and only mild side effects, warranting further studies to evaluate its potential for future use as a safe, tolerable anti-HBV medication. No significant differences were noted amongst trials included in either of Vesatolimod doses (Vesatolimod 1 mg, RR = 0.99, 95% CI 0.76-1.30, P = .95, I2 = 0%; Vesatolimod 2 mg, RR = 1.06, 95% CI 0.82-1.37, P = .66, I2 = 0%; Vesatolimod 4 mg, RR = 1.06, 95% CI 0.82-1.37, P = .66, I2 = 0%;), further suggesting its comparable safety in comparison to oral antiviral agents.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures






References
-
- World Health Organization. Hepatitis B. World Health Organization. 2001. Retrieved May 1, 2022, from. Available at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
-
- Shih C, Yang CC, Choijilsuren G, et al. Hepatitis B virus. Trends Microbiol. 2018;26:386–7. - PubMed
-
- Centers for Disease Control and Prevention. Hepatitis B - faqs, Statistics, data, & guidelines. Centers for Disease Control and Prevention. 2023, March 9. Retrieved May 1, 2022, from. Available at: https://www.cdc.gov/hepatitis/hbv/index.htm#:~:text=Hepatitis%20B%20is%2....
-
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

