An ImageJ macro tool for OCTA-based quantitative analysis of Myopic Choroidal neovascularization
- PMID: 37083836
- PMCID: PMC10120933
- DOI: 10.1371/journal.pone.0283929
An ImageJ macro tool for OCTA-based quantitative analysis of Myopic Choroidal neovascularization
Abstract
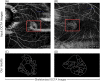
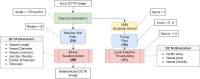
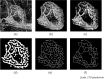
Myopic Choroidal neovascularization (mCNV) is one of the most common vision-threatening com- plications of pathological myopia among many retinal diseases. Optical Coherence Tomography Angiography (OCTA) is an emerging newer non-invasive imaging technique and is recently being included in the investigation and treatment of mCNV. However, there exists no standard tool for time-efficient and dependable analysis of OCTA images of mCNV. In this study, we propose a customizable ImageJ macro that automates the OCTA image processing and lets users measure nine mCNV biomarkers. We developed a three-stage image processing pipeline to process the OCTA images using the macro. The images were first manually delineated, and then denoised using a Gaussian Filter. This was followed by the application of the Frangi filter and Local Adaptive thresholding. Finally, skeletonized images were obtained using the Mexican Hat filter. Nine vascular biomarkers including Junction Density, Vessel Diameter, and Fractal Dimension were then computed from the skeletonized images. The macro was tested on a 26 OCTA image dataset for all biomarkers. Two trends emerged in the computed biomarker values. First, the lesion-size dependent parameters (mCNV Area (mm2) Mean = 0.65, SD = 0.46) showed high variation, whereas normalized parameters (Junction Density(n/mm): Mean = 10.24, SD = 0.63) were uniform throughout the dataset. The computed values were consistent with manual measurements within existing literature. The results illustrate our ImageJ macro to be a convenient alternative for manual OCTA image processing, including provisions for batch processing and parameter customization, providing a systematic, reliable analysis of mCNV.
Copyright: © 2023 Deshpande et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources

