Bioinspired figure-ground discrimination via visual motion smoothing
- PMID: 37083880
- PMCID: PMC10155969
- DOI: 10.1371/journal.pcbi.1011077
Bioinspired figure-ground discrimination via visual motion smoothing
Abstract
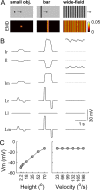
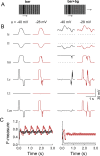
Flies detect and track moving targets among visual clutter, and this process mainly relies on visual motion. Visual motion is analyzed or computed with the pathway from the retina to T4/T5 cells. The computation of local directional motion was formulated as an elementary movement detector (EMD) model more than half a century ago. Solving target detection or figure-ground discrimination problems can be equivalent to extracting boundaries between a target and the background based on the motion discontinuities in the output of a retinotopic array of EMDs. Individual EMDs cannot measure true velocities, however, due to their sensitivity to pattern properties such as luminance contrast and spatial frequency content. It remains unclear how local directional motion signals are further integrated to enable figure-ground discrimination. Here, we present a computational model inspired by fly motion vision. Simulations suggest that the heavily fluctuating output of an EMD array is naturally surmounted by a lobula network, which is hypothesized to be downstream of the local motion detectors and have parallel pathways with distinct directional selectivity. The lobula network carries out a spatiotemporal smoothing operation for visual motion, especially across time, enabling the segmentation of moving figures from the background. The model qualitatively reproduces experimental observations in the visually evoked response characteristics of one type of lobula columnar (LC) cell. The model is further shown to be robust to natural scene variability. Our results suggest that the lobula is involved in local motion-based target detection.
Copyright: © 2023 Wu, Guo. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









Similar articles
-
Moving object detection based on bioinspired background subtraction.Bioinspir Biomim. 2024 Jul 8;19(5). doi: 10.1088/1748-3190/ad5ba3. Bioinspir Biomim. 2024. PMID: 38917814
-
A directional tuning map of Drosophila elementary motion detectors.Nature. 2013 Aug 8;500(7461):212-6. doi: 10.1038/nature12320. Nature. 2013. PMID: 23925246
-
The computational basis of an identified neuronal circuit for elementary motion detection in dipterous insects.Vis Neurosci. 2004 Jul-Aug;21(4):567-86. doi: 10.1017/S0952523804214079. Vis Neurosci. 2004. PMID: 15579222
-
Common circuit design in fly and mammalian motion vision.Nat Neurosci. 2015 Aug;18(8):1067-76. doi: 10.1038/nn.4050. Epub 2015 Jun 29. Nat Neurosci. 2015. PMID: 26120965 Review.
-
Visual Circuits for Direction Selectivity.Annu Rev Neurosci. 2017 Jul 25;40:211-230. doi: 10.1146/annurev-neuro-072116-031335. Epub 2017 Apr 18. Annu Rev Neurosci. 2017. PMID: 28418757 Free PMC article. Review.
Cited by
-
Research on motion target detection based on infrared biomimetic compound eye camera.Sci Rep. 2024 Nov 11;14(1):27519. doi: 10.1038/s41598-024-78790-9. Sci Rep. 2024. PMID: 39528635 Free PMC article.
References
-
- Dvorak DR, Bishop LG, Eckert HE. On the identification of movement detectors in the fly optic lobe. J comp Physiol. 1975; 100:5–23. 10.1007/BF00623928 - DOI
-
- Hausen K. Functional characterization and anatomical identification of motion sensitive neurons in the lobula plate of the blowfly Calliphora erythrocephala. Z Naturforsch. 1976; 31c:629–633.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

