Hematopoietic NLRP3 and AIM2 Inflammasomes Promote Diabetes-Accelerated Atherosclerosis, but Increased Necrosis Is Independent of Pyroptosis
- PMID: 37083999
- PMCID: PMC10281813
- DOI: 10.2337/db22-0962
Hematopoietic NLRP3 and AIM2 Inflammasomes Promote Diabetes-Accelerated Atherosclerosis, but Increased Necrosis Is Independent of Pyroptosis
Abstract
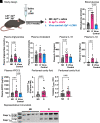
Serum apolipoprotein C3 (APOC3) predicts incident cardiovascular events in people with type 1 diabetes, and silencing of APOC3 prevents both lesion initiation and advanced lesion necrotic core expansion in a mouse model of type 1 diabetes. APOC3 acts by slowing the clearance of triglyceride-rich lipoproteins, but lipid-free APOC3 has recently been reported to activate an inflammasome pathway in monocytes. We therefore investigated the contribution of hematopoietic inflammasome pathways to atherosclerosis in mouse models of type 1 diabetes. LDL receptor-deficient diabetes mouse models were transplanted with bone marrow from donors deficient in NOD, LRR and pyrin domain-containing protein 3 (NLRP3), absent in melanoma 2 (AIM2) or gasdermin D (GSDMD), an inflammasome-induced executor of pyroptotic cell death. Mice with diabetes exhibited inflammasome activation and consistently, increased plasma interleukin-1β (IL-1β) and IL-18. Hematopoietic deletions of NLRP3, AIM2, or GSDMD caused smaller atherosclerotic lesions in diabetic mice. The increased lesion necrotic core size in diabetic mice was independent of macrophage pyroptosis because hematopoietic GSDMD deficiency failed to prevent necrotic core expansion in advanced lesions. Our findings demonstrate that AIM2 and NLRP3 inflammasomes contribute to atherogenesis in diabetes and suggest that necrotic core expansion is independent of macrophage pyroptosis.
Article highlights: The contribution of hematopoietic cell inflammasome activation to atherosclerosis associated with type 1 diabetes is unknown. The goal of this study was to address whether hematopoietic NOD, LRR, and pyrin domain-containing protein 3 (NLRP3), absent in melanoma 2 (AIM2) inflammasomes, or the pyroptosis executioner gasdermin D (GSDMD) contributes to atherosclerosis in mouse models of type 1 diabetes. Diabetic mice exhibited increased inflammasome activation, with hematopoietic deletions of NLRP3, AIM2, or GSDMD causing smaller atherosclerotic lesions in diabetic mice, but the increased lesion necrotic core size in diabetic mice was independent of macrophage pyroptosis. Further studies on whether inflammasome activation contributes to cardiovascular complications in people with type 1 diabetes are warranted.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures





References
-
- Tsao CW, Aday AW, Almarzooq ZI, et al. . Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation 2022;145:e153–e639 - PubMed
-
- Nishikawa T, Edelstein D, Du XL, et al. . Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787–790 - PubMed
-
- Sharma A, Choi JSY, Stefanovic N, et al. . Specific NLRP3 inhibition protects against diabetes-associated atherosclerosis. Diabetes 2021;70:772–787 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

