A Mixture Model for Estimating SARS-CoV-2 Seroprevalence in Chennai, India
- PMID: 37084085
- PMCID: PMC10472327
- DOI: 10.1093/aje/kwad103
A Mixture Model for Estimating SARS-CoV-2 Seroprevalence in Chennai, India
Abstract
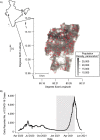
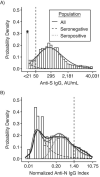
Serological assays used to estimate the prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) often rely on manufacturers' cutoffs established on the basis of severe cases. We conducted a household-based serosurvey of 4,677 individuals in Chennai, India, from January to May 2021. Samples were tested for SARS-CoV-2 immunoglobulin G (IgG) antibodies to the spike (S) and nucleocapsid (N) proteins. We calculated seroprevalence, defining seropositivity using manufacturer cutoffs and using a mixture model based on measured IgG level. Using manufacturer cutoffs, there was a 5-fold difference in seroprevalence estimated by each assay. This difference was largely reconciled using the mixture model, with estimated anti-S and anti-N IgG seroprevalence of 64.9% (95% credible interval (CrI): 63.8, 66.0) and 51.5% (95% CrI: 50.2, 52.9), respectively. Age and socioeconomic factors showed inconsistent relationships with anti-S and anti-N IgG seropositivity using manufacturer cutoffs. In the mixture model, age was not associated with seropositivity, and improved household ventilation was associated with lower seropositivity odds. With global vaccine scale-up, the utility of the more stable anti-S IgG assay may be limited due to the inclusion of the S protein in several vaccines. Estimates of SARS-CoV-2 seroprevalence using alternative targets must consider heterogeneity in seroresponse to ensure that seroprevalence is not underestimated and correlates are not misinterpreted.
Keywords: COVID-19; India; SARS-CoV-2; coronavirus disease 2019; mixture models; seroprevalence; serosurveys; severe acute respiratory syndrome coronavirus 2.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health.
Figures

References
-
- O’Driscoll M, Dos Santos GR, Wang L, et al. . Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

