The Promise of Niacin in Neurology
- PMID: 37084148
- PMCID: PMC10457276
- DOI: 10.1007/s13311-023-01376-2
The Promise of Niacin in Neurology
Abstract
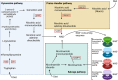
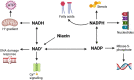
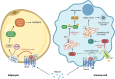
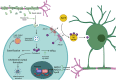
Niacin (vitamin B3) is an essential nutrient that treats pellagra, and prior to the advent of statins, niacin was commonly used to counter dyslipidemia. Recent evidence has posited niacin as a promising therapeutic for several neurological disorders. In this review, we discuss the biochemistry of niacin, including its homeostatic roles in NAD+ supplementation and metabolism. Niacin also has roles outside of metabolism, largely through engaging hydroxycarboxylic acid receptor 2 (Hcar2). These receptor-mediated activities of niacin include regulation of immune responses, phagocytosis of myelin debris after demyelination or of amyloid beta in models of Alzheimer's disease, and cholesterol efflux from cells. We describe the neurological disorders in which niacin has been investigated or has been proposed as a candidate medication. These are multiple sclerosis, Alzheimer's disease, Parkinson's disease, glioblastoma and amyotrophic lateral sclerosis. Finally, we explore the proposed mechanisms through which niacin may ameliorate neuropathology. While several questions remain, the prospect of niacin as a therapeutic to alleviate neurological impairment is promising.
Keywords: Hydroxycarboxylic acid receptor (Hcar)2; Immunomodulation; NAD+/NADP; Neurological diseases; Niacin treatment; Phagocytosis.
© 2023. The American Society for Experimental Neurotherapeutics, Inc.
Figures
Similar articles
-
Chapter 30: historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins.Handb Clin Neurol. 2010;95:445-76. doi: 10.1016/S0072-9752(08)02130-1. Handb Clin Neurol. 2010. PMID: 19892133 Review.
-
Niacin in the Central Nervous System: An Update of Biological Aspects and Clinical Applications.Int J Mol Sci. 2019 Feb 23;20(4):974. doi: 10.3390/ijms20040974. Int J Mol Sci. 2019. PMID: 30813414 Free PMC article. Review.
-
The niacin receptor HCAR2 modulates microglial response and limits disease progression in a mouse model of Alzheimer's disease.Sci Transl Med. 2022 Mar 23;14(637):eabl7634. doi: 10.1126/scitranslmed.abl7634. Epub 2022 Mar 23. Sci Transl Med. 2022. PMID: 35320002 Free PMC article.
-
[Niacin deficiency and cutaneous immunity].Nihon Rinsho Meneki Gakkai Kaishi. 2015;38(1):37-44. doi: 10.2177/jsci.38.37. Nihon Rinsho Meneki Gakkai Kaishi. 2015. PMID: 25765687 Review. Japanese.
-
NADP+ biosynthesis by rats receiving a pellagragenic diet.Braz J Med Biol Res. 1991;24(6):563-6. Braz J Med Biol Res. 1991. PMID: 1823271
Cited by
-
Is dolichol pathway dysfunction a significant factor in Alzheimer's disease?Inflammopharmacology. 2025 Jul 25. doi: 10.1007/s10787-025-01868-x. Online ahead of print. Inflammopharmacology. 2025. PMID: 40715929
-
Clinical Insights on Caloric Restriction Mimetics for Mitigating Brain Aging and Related Neurodegeneration.Cell Mol Neurobiol. 2024 Oct 16;44(1):67. doi: 10.1007/s10571-024-01493-2. Cell Mol Neurobiol. 2024. PMID: 39412683 Free PMC article. Review.
-
Association of niacin intake and metabolic dysfunction-associated steatotic liver disease: findings from National Health and Nutrition Examination Survey.BMC Public Health. 2024 Oct 8;24(1):2742. doi: 10.1186/s12889-024-20161-0. BMC Public Health. 2024. PMID: 39379884 Free PMC article.
-
Comparative Proteomics Highlights that GenX Exposure Leads to Metabolic Defects and Inflammation in Astrocytes.Environ Sci Technol. 2024 Nov 19;58(46):20525-20539. doi: 10.1021/acs.est.4c05472. Epub 2024 Nov 5. Environ Sci Technol. 2024. PMID: 39499804 Free PMC article.
-
The Role of Nicotinamide as Chemo-Preventive Agent in NMSCs: A Systematic Review and Meta-Analysis.Nutrients. 2023 Dec 27;16(1):100. doi: 10.3390/nu16010100. Nutrients. 2023. PMID: 38201930 Free PMC article.
References
-
- Mielgo-Ayuso JA-O, Aparicio-Ugarriza R, Olza JA-O, Aranceta-Bartrina J, Gil Á A-O, Ortega RA-O, et al. Dietary intake and food sources of niacin, riboflavin, thiamin and vitamin B6 in a representative sample of the Spanish population. The anthropometry, intake, and energy balance in Spain (ANIBES) study †. Nutrients. 2018;10(2072–6643 (Electronic)):846. - PMC - PubMed
-
- Hołubiec P, Leończyk M, Staszewski F, Łazarczyk A, Jaworek AK, Rojas-Pelc A. Pathophysiology and clinical management of pellagra - a review. Folia Med Crac. 2021;61(0015–5616 (Print)):125–37. - PubMed
-
- Boden WE, Sidhu MS, Toth PP. The therapeutic role of niacin in dyslipidemia management. J Cardiovasc Pharmacol Ther. 2014;19(1940–4034 (Electronic)):141–58. - PubMed
-
- Organization WH. Pellagra and its prevention and control in major emergencies. 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

