An Open Label, Randomized, Multicenter Study of Elafibranor in Children With Nonalcoholic Steatohepatitis
- PMID: 37084342
- PMCID: PMC10523882
- DOI: 10.1097/MPG.0000000000003796
An Open Label, Randomized, Multicenter Study of Elafibranor in Children With Nonalcoholic Steatohepatitis
Abstract
Objectives: Nonalcoholic fatty liver disease is the most common chronic liver disease in children. Elafibranor, a dual peroxisome proliferator-activated receptor α/δ agonist, has been proposed as a treatment for nonalcoholic steatohepatitis (NASH). The aims were to (1) describe pharmacokinetics (PK), safety, and tolerability of oral elafibranor at 2 doses (80 and 120 mg) in children 8-17 years and (2) assess changes in aminotransferases.
Methods: Children with NASH were randomized to open-label elafibranor 80 mg or 120 mg daily for 12 weeks. The intent-to-treat analysis included all participants who received at least 1 dose. Standard descriptive statistics and PK analyses were performed.
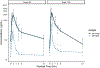
Results: Ten males [mean 15.1 years, standard deviation (SD) 2.2] with NASH were randomized to 80 mg (n = 5) or 120 mg (n = 5). Baseline mean alanine aminotransferase (ALT) was 82 U/L (SD 13) and 87 U/L (SD 20) for 80 mg and 120 mg groups, respectively. Elafibranor was rapidly absorbed and well tolerated. Elafibranor plasma exposure increased between the 80 mg and 120 mg dose with a 1.9- and 1.3-fold increase in median Cmax and AUC 0-24 , respectively. End of treatment mean ALT was 52 U/L (SD 20) for the 120 mg group, with a relative mean ALT change from baseline of -37.4% (SD 23.8%) at 12 weeks.
Conclusions: Once daily dosing of elafibranor was well tolerated in children with NASH. There was a 37.4% relative reduction from mean baseline ALT in the 120 mg group. Decreasing ALT may be associated with improvement in liver histology, thus could be considered a surrogate for histology in early phase trials. These results may support further exploration of elafibranor in children with NASH.
Trial registration: ClinicalTrials.gov NCT03883607.
Copyright © 2023 by European Society for European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Conflict of interest statement
B.N. and D.M. are shareholders of GENFIT. J.B.S. reports grants to UCSD from Intercept and Seraphina outside the submitted work. The remaining authors report no conflicts of interest.
Figures

References
-
- Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–1393. - PubMed
-
- Alkhouri N. Liver Transplantation for Nonalcoholic Steatohepatitis (NASH) in Children and Young Adults: The True Burden of Pediatric Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;148(4):S–1046.
-
- Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005. Sep;42(3):641–9. - PubMed
-
- Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150(5):1147–1159.e5. - PubMed

