Burosumab vs Phosphate/Active Vitamin D in Pediatric X-Linked Hypophosphatemia: A Subgroup Analysis by Dose Level
- PMID: 37084401
- PMCID: PMC10583998
- DOI: 10.1210/clinem/dgad230
Burosumab vs Phosphate/Active Vitamin D in Pediatric X-Linked Hypophosphatemia: A Subgroup Analysis by Dose Level
Abstract
Context: In an open-label, randomized, controlled, phase 3 trial in 61 children aged 1 to 12 years with X-linked hypophosphatemia (XLH), burosumab improved rickets vs continuing conventional therapy with active vitamin D and phosphate.
Objective: We conducted an analysis to determine whether skeletal responses differed when switching to burosumab vs continuing higher or lower doses of conventional therapy.
Methods: Conventional therapy dose groups were defined as higher-dose phosphate [greater than 40 mg/kg] (HPi), lower-dose phosphate [40 mg/kg or less] (LPi), higher-dose alfacalcidol [greater than 60 ng/kg] or calcitriol [greater than 30 ng/kg] (HD), and lower-dose alfacalcidol [60 ng/kg or less] or calcitriol [30 ng/kg or less] (LD).
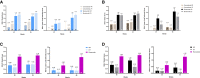
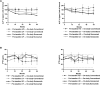
Results: At week 64, the Radiographic Global Impression of Change (RGI-C) for rickets was higher (better) in children randomly assigned to burosumab vs conventional therapy for all prebaseline dose groups: HPi (+1.72 vs +0.67), LPi (+2.14 vs +1.08), HD (+1.90 vs +0.94), LD (+2.11 vs +1.06). At week 64, the RGI-C for rickets was also higher in children randomly assigned to burosumab (+2.06) vs conventional therapy for all on-study dose groups: HPi (+1.03), LPi (+1.05), HD (+1.45), LD (+0.72). Serum alkaline phosphatase (ALP) also decreased in the burosumab-treated patients more than in the conventional therapy group, regardless of on-study phosphate and active vitamin D doses.
Conclusion: Prior phosphate or active vitamin D doses did not influence treatment response after switching to burosumab among children with XLH and active radiographic rickets. Switching from conventional therapy to burosumab improved rickets and serum ALP more than continuing either higher or lower doses of phosphate or active vitamin D.
Trial registration: ClinicalTrials.gov NCT02915705.
Keywords: FGF23; X-linked hypophosphatemia; XLH; active vitamin D; burosumab; oral phosphate; rickets.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
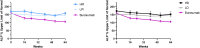
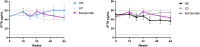
References
-
- Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M. Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med. 1991;325(26):1843‐1848. - PubMed
-
- Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987‐1998. - PubMed
-
- Whyte MP, Carpenter TO, Gottesman GS, et al. Efficacy and safety of burosumab in children aged 1-4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7(3):189‐199. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

