Very-long-chain fatty acids induce glial-derived sphingosine-1-phosphate synthesis, secretion, and neuroinflammation
- PMID: 37084732
- PMCID: PMC10160010
- DOI: 10.1016/j.cmet.2023.03.022
Very-long-chain fatty acids induce glial-derived sphingosine-1-phosphate synthesis, secretion, and neuroinflammation
Abstract
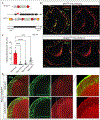
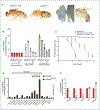
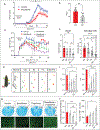
VLCFAs (very-long-chain fatty acids) are the most abundant fatty acids in myelin. Hence, during demyelination or aging, glia are exposed to higher levels of VLCFA than normal. We report that glia convert these VLCFA into sphingosine-1-phosphate (S1P) via a glial-specific S1P pathway. Excess S1P causes neuroinflammation, NF-κB activation, and macrophage infiltration into the CNS. Suppressing the function of S1P in fly glia or neurons, or administration of Fingolimod, an S1P receptor antagonist, strongly attenuates the phenotypes caused by excess VLCFAs. In contrast, elevating the VLCFA levels in glia and immune cells exacerbates these phenotypes. Elevated VLCFA and S1P are also toxic in vertebrates based on a mouse model of multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE). Indeed, reducing VLCFA with bezafibrate ameliorates the phenotypes. Moreover, simultaneous use of bezafibrate and fingolimod synergizes to improve EAE, suggesting that lowering VLCFA and S1P is a treatment avenue for MS.
Keywords: NF-κB activation; VLCFA β-oxidation; fingolimod; lipid metabolism; multiple sclerosis; myelin lipid; neurodegeneration; neuroinflammation; sphingolipid; sphingosine 1-phosphate.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

