Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study
- PMID: 37084748
- PMCID: PMC10408380
- DOI: 10.1016/S1470-2045(23)00146-8
Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study
Abstract
Background: Compared with photon therapy, proton therapy reduces exposure of normal brain tissue in patients with craniopharyngioma, which might reduce cognitive deficits associated with radiotherapy. Because there are known physical differences between the two methods of radiotherapy, we aimed to estimate progression-free survival and overall survival distributions for paediatric and adolescent patients with craniopharyngioma treated with limited surgery and proton therapy, while monitoring for excessive CNS toxicity.
Methods: In this single-arm, phase 2 study, patients with craniopharyngioma at St Jude Children's Research Hospital (Memphis TN, USA) and University of Florida Health Proton Therapy Institute (Jacksonville, FL, USA) were recruited. Patients were eligible if they were aged 0-21 years at the time of enrolment and had not been treated with previous radiotherapeutic or intracystic therapies. Eligible patients were treated using passively scattered proton beams, 54 Gy (relative biological effect), and a 0·5 cm clinical target volume margin. Surgical treatment was individualised before proton therapy and included no surgery, single procedures with catheter and Ommaya reservoir placement through a burr hole or craniotomy, endoscopic resection, trans-sphenoidal resection, craniotomy, or multiple procedure types. After completing treatment, patients were evaluated clinically and by neuroimaging for tumour progression and evidence of necrosis, vasculopathy, permanent neurological deficits, vision loss, and endocrinopathy. Neurocognitive tests were administered at baseline and once a year for 5 years. Outcomes were compared with a historical cohort treated with surgery and photon therapy. The coprimary endpoints were progression-free survival and overall survival. Progression was defined as an increase in tumour dimensions on successive imaging evaluations more than 2 years after treatment. Survival and safety were also assessed in all patients who received photon therapy and limited surgery. This study is registered with ClinicalTrials.gov, NCT01419067.
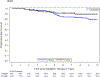
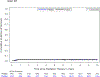
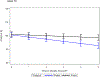
Findings: Between Aug 22, 2011, and Jan 19, 2016, 94 patients were enrolled and treated with surgery and proton therapy, of whom 49 (52%) were female, 45 (48%) were male, 62 (66%) were White, 16 (17%) were Black, two (2%) were Asian, and 14 (15%) were other races, and median age was 9·39 years (IQR 6·39-13·38) at the time of radiotherapy. As of data cutoff (Feb 2, 2022), median follow-up was 7·52 years (IQR 6·28-8·53) for patients who did not have progression and 7·62 years (IQR 6·48-8·54) for the full cohort of 94 patients. 3-year progression-free survival was 96·8% (95% CI 90·4-99·0; p=0·89), with progression occurring in three of 94 patients. No deaths occurred at 3 years, such that overall survival was 100%. At 5 years, necrosis had occurred in two (2%) of 94 patients, severe vasculopathy in four (4%), and permanent neurological conditions in three (3%); decline in vision from normal to abnormal occurred in four (7%) of 54 patients with normal vision at baseline. The most common grade 3-4 adverse events were headache (six [6%] of 94 patients), seizure (five [5%]), and vascular disorders (six [6%]). No deaths occurred as of data cutoff.
Interpretation: Proton therapy did not improve survival outcomes in paediatric and adolescent patients with craniopharyngioma compared with a historical cohort, and severe complication rates were similar. However, cognitive outcomes with proton therapy were improved over photon therapy. Children and adolescents treated for craniopharyngioma using limited surgery and post-operative proton therapy have a high rate of tumour control and low rate of severe complications. The outcomes achieved with this treatment represent a new benchmark to which other regimens can be compared.
Funding: American Lebanese Syrian Associated Charities, American Cancer Society, the US National Cancer Institute, and Research to Prevent Blindness.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests
Figures






Comment in
-
Craniopharyngiomas and proton beam therapy: worth the expense?Lancet Oncol. 2023 May;24(5):422-423. doi: 10.1016/S1470-2045(23)00162-6. Epub 2023 Apr 18. Lancet Oncol. 2023. PMID: 37084749 No abstract available.
References
-
- Muller HL, Merchant TE, Puget S, Martinez-Barbera JP. New outlook on the diagnosis, treatment and follow-up of childhood-onset craniopharyngioma. Nat Rev Endocrinol 2017; 13(5): 299–312. - PubMed
-
- Muller HL. Childhood craniopharyngioma: current controversies on management in diagnostics, treatment and follow-up. Expert Rev Neurother 2010; 10(4): 515–24. - PubMed
-
- Hill TK, Baine MJ, Verma V, et al. Patterns of Care in Pediatric Craniopharyngioma: Outcomes Following Definitive Radiotherapy. Anticancer Res 2019; 39(2): 803–7. - PubMed
-
- Maarouf M, El Majdoub F, Fuetsch M, et al. Stereotactic intracavitary brachytherapy with P-32 for cystic craniopharyngiomas in children. Strahlenther Onkol 2016; 192(3): 157–65. - PubMed
-
- Suh JH, Gupta N. Role of radiation therapy and radiosurgery in the management of craniopharyngiomas. Neurosurg Clin N Am 2006; 17(2): 143–8, vi-vii. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

