Prenatal benzene exposure in mice alters offspring hypothalamic development predisposing to metabolic disease in later life
- PMID: 37084897
- PMCID: PMC10199724
- DOI: 10.1016/j.chemosphere.2023.138738
Prenatal benzene exposure in mice alters offspring hypothalamic development predisposing to metabolic disease in later life
Abstract
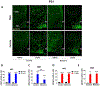
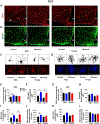
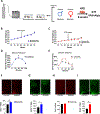
Maternal exposure to environmental contaminants during pregnancy poses a significant threat to a developing fetus, as these substances can easily cross the placenta and disrupt the neurodevelopment of offspring. Specifically, the hypothalamus is essential in the regulation of metabolism, notably during critical windows of development. An abnormal hormonal and inflammatory milieu during development can trigger persistent changes in the function of hypothalamic circuits, leading to long-lasting effects on the body's energy homeostasis and metabolism. We recently demonstrated that gestational exposure to clinically relevant levels of benzene induces severe metabolic dysregulation in the offspring. Given the central role of the hypothalamus in metabolic control, we hypothesized that prenatal exposure to benzene impacts hypothalamic development, contributing to the adverse metabolic effects in the offspring. C57BL/6JB dams were exposed to benzene at 50 ppm in the inhalation chambers exclusively during pregnancy (from E0.5 to E19). Transcriptomic analysis of the exposed offspring at postnatal day 21 (P21) revealed hypothalamic changes in genes related to metabolic regulation, inflammation, and neurodevelopment exclusively in males. Moreover, the hypothalamus of prenatally benzene-exposed male offspring displayed alterations in orexigenic and anorexigenic projections, impairments in leptin signaling, and increased microgliosis. Additional exposure to benzene during lactation did not promote further microgliosis or astrogliosis in the offspring, while the high-fat diet (HFD) challenge in adulthood exacerbated glucose metabolism and hypothalamic inflammation in benzene-exposed offspring of both sexes. These findings reveal the persistent adverse effects of prenatal benzene exposure on hypothalamic circuits and neuroinflammation, predisposing the offspring to long-lasting metabolic health conditions.
Keywords: Air pollution; Hypothalamic development; Metabolic programming; Metabolic syndrome; Neuroinflammation; Prenatal exposures.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Update of
-
Prenatal benzene exposure alters offspring hypothalamic development predisposing to metabolic disease in later life.bioRxiv [Preprint]. 2023 Jan 5:2023.01.05.522910. doi: 10.1101/2023.01.05.522910. bioRxiv. 2023. Update in: Chemosphere. 2023 Jul;330:138738. doi: 10.1016/j.chemosphere.2023.138738. PMID: 36711607 Free PMC article. Updated. Preprint.
Similar articles
-
Prenatal benzene exposure alters offspring hypothalamic development predisposing to metabolic disease in later life.bioRxiv [Preprint]. 2023 Jan 5:2023.01.05.522910. doi: 10.1101/2023.01.05.522910. bioRxiv. 2023. Update in: Chemosphere. 2023 Jul;330:138738. doi: 10.1016/j.chemosphere.2023.138738. PMID: 36711607 Free PMC article. Updated. Preprint.
-
Effects of maternal high-fat diet on the hypothalamic components related to food intake and energy expenditure in mice offspring.Life Sci. 2022 Oct 15;307:120880. doi: 10.1016/j.lfs.2022.120880. Epub 2022 Aug 10. Life Sci. 2022. PMID: 35963301
-
Developmental exposure to indoor flame retardants and hypothalamic molecular signatures: Sex-dependent reprogramming of lipid homeostasis.Front Endocrinol (Lausanne). 2022 Sep 30;13:997304. doi: 10.3389/fendo.2022.997304. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36277707 Free PMC article.
-
Body Fat Distribution and Glucose Homeostasis Is Affected by Perinatal Exposure to High Dietary Advanced Glycation End Products (AGEs) in Male Offspring.Am J Reprod Immunol. 2025 Apr;93(4):e70073. doi: 10.1111/aji.70073. Am J Reprod Immunol. 2025. PMID: 40190117
-
Glial adaptations to high-fat diet in the mediobasal hypothalamus and effects on metabolic control.Physiol Behav. 2025 Oct 15;300:115017. doi: 10.1016/j.physbeh.2025.115017. Epub 2025 Jul 7. Physiol Behav. 2025. PMID: 40633635 Review.
Cited by
-
Adult-Onset Transcriptomic Effects of Developmental Exposure to Benzene in Zebrafish (Danio rerio): Evaluating a Volatile Organic Compound of Concern.Int J Mol Sci. 2023 Nov 11;24(22):16212. doi: 10.3390/ijms242216212. Int J Mol Sci. 2023. PMID: 38003401 Free PMC article.
-
Microglial ER stress response via IRE1α regulates diet-induced metabolic imbalance and obesity in mice.Mol Metab. 2025 May;95:102128. doi: 10.1016/j.molmet.2025.102128. Epub 2025 Mar 20. Mol Metab. 2025. PMID: 40120978 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

