Antiviral Treatment of Coronavirus Disease-2019 Pneumonia
- PMID: 37085220
- PMCID: PMC9701636
- DOI: 10.1016/j.ccm.2022.11.008
Antiviral Treatment of Coronavirus Disease-2019 Pneumonia
Abstract
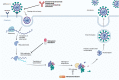
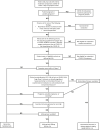
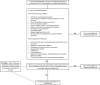
Direct acting antivirals and monoclonal antibodies reduce morbidity and mortality associated with severe acute respiratory syndrome coronavirus 2 infection. Persons at higher risk for disease progression and hospitalized patients with coronavirus disease-2019 (COVID-19) benefit most from available therapies. Following an emphasis on inpatient treatment of COVID-19 during the early pandemic, several therapeutic options were developed for outpatients with COVID-19. Additional clinical trials and real-world studies are needed to keep pace with the evolving pandemic.
Keywords: Bebtelovimab; COVID-19; Molnupiravir; Nirmatrelvir; Remdesivir; SARS-CoV-2; Tixagevimab/cilgavimab.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures
References
-
- WHO. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ Available at: Accessed September 30, 2022.
-
- Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate covid-19. N Engl J Med. 2020;383(18):1757–1766. (In eng) - PubMed
-
- Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N Engl J Med. 2020;383(25):2451–2460. (In eng) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

