Machine learning identifies clusters of longitudinal autoantibody profiles predictive of systemic lupus erythematosus disease outcomes
- PMID: 37085289
- PMCID: PMC11293954
- DOI: 10.1136/ard-2022-223808
Machine learning identifies clusters of longitudinal autoantibody profiles predictive of systemic lupus erythematosus disease outcomes
Abstract
Objectives: A novel longitudinal clustering technique was applied to comprehensive autoantibody data from a large, well-characterised, multinational inception systemic lupus erythematosus (SLE) cohort to determine profiles predictive of clinical outcomes.
Methods: Demographic, clinical and serological data from 805 patients with SLE obtained within 15 months of diagnosis and at 3-year and 5-year follow-up were included. For each visit, sera were assessed for 29 antinuclear antibodies (ANA) immunofluorescence patterns and 20 autoantibodies. K-means clustering on principal component analysis-transformed longitudinal autoantibody profiles identified discrete phenotypic clusters. One-way analysis of variance compared cluster enrolment demographics and clinical outcomes at 10-year follow-up. Cox proportional hazards model estimated the HR for survival adjusting for age of disease onset.
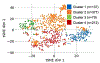
Results: Cluster 1 (n=137, high frequency of anti-Smith, anti-U1RNP, AC-5 (large nuclear speckled pattern) and high ANA titres) had the highest cumulative disease activity and immunosuppressants/biologics use at year 10. Cluster 2 (n=376, low anti-double stranded DNA (dsDNA) and ANA titres) had the lowest disease activity, frequency of lupus nephritis and immunosuppressants/biologics use. Cluster 3 (n=80, highest frequency of all five antiphospholipid antibodies) had the highest frequency of seizures and hypocomplementaemia. Cluster 4 (n=212) also had high disease activity and was characterised by multiple autoantibody reactivity including to antihistone, anti-dsDNA, antiribosomal P, anti-Sjögren syndrome antigen A or Ro60, anti-Sjögren syndrome antigen B or La, anti-Ro52/Tripartite Motif Protein 21, antiproliferating cell nuclear antigen and anticentromere B). Clusters 1 (adjusted HR 2.60 (95% CI 1.12 to 6.05), p=0.03) and 3 (adjusted HR 2.87 (95% CI 1.22 to 6.74), p=0.02) had lower survival compared with cluster 2.
Conclusion: Four discrete SLE patient longitudinal autoantibody clusters were predictive of long-term disease activity, organ involvement, treatment requirements and mortality risk.
Keywords: autoantibodies; autoimmunity; systemic lupus erythematosus.
© Author(s) (or their employer(s)) 2023. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MYC has received consulting fees from Janssen, AstraZeneca, Mallinckrodt Pharmaceuticals and MitogenDx. AEC has received consulting fees, speaking fees and/or honoraria from AstraZeneca, Bristol Myers Squibb and GlaxoSmithKline (<US$10 000 each) and research support from GlaxoSmithKline. MJF is Director of Mitogen Diagnostics (Calgary, Alberta, Canada) and a consultant to Werfen International (San Diego, California, USA; Barcelona, Spain), Aesku Group (Wendelsheim, Germany) and Alexion Canada (<US$10 000). CG has received consulting fees, speaking fees and/or honoraria from Eli Lilly, UCB, GlaxoSmithKline, Merck Serono and BMS (<US$10 000 each) and grants from UCB. Grants from UCB were not to CG but to Sandwell and West Birmingham Hospitals NHS Trust. DDG received consulting fees, speaking fees and/or honoraria from GlaxoSmithKline (<US$10 000). INB has received consulting fees, speaking fees and/or honoraria from Eli Lilly, UCB, Roche, Merck Serono, MedImmune (<US$10 000 each) and grants from UCB, Genzyme Sanofi and GlaxoSmithKline. EMG has paid consultation with investment analysts Guidepoint Global Gerson Lerman Group. KK has received grants from UCB, Human Genome Sciences/GlaxoSmithKline, Takeda, Ablynx, Bristol Myers Squibb, Pfizer and Kyowa Hakko Kirin, and has received consulting fees from Exagen Diagnostics, Genentech, Eli Lilly, Bristol Myers Squibb and Anthera (<US$10 000 each). KHC has consulted for or collaborated on research projects with Janssen, GlaxoSmithKline, Gilead, Exagen Diagnostics, Lilly, Merck, AstraZeneca, Amgen and Neutrolis (<US$10 000 each). The remainder of the authors have no disclosures.
Figures

References
-
- Budde P, Zucht HD, Vordenbäumen S, Goehler H, Fischer-Betz R, Gamer M, et al. Multiparametric detection of autoantibodies in systemic lupus erythematosus. Lupus. 2016;25(8):812–22. - PubMed
-
- To CH, Petri M. Is antibody clustering predictive of clinical subsets and damage in systemic lupus erythematosus? Arthritis Rheum. 2005;52(12):4003–10. - PubMed
-
- Tápanes FJ, Vásquez M, Ramírez R, Matheus C, Rodríguez MA, Bianco N. Cluster analysis of antinuclear autoantibodies in the prognosis of SLE nephropathy: are anti-extractable nuclear antibodies protective? Lupus. 2000;9(6):437–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR046588/AR/NIAMS NIH HHS/United States
- M01 RR000046/RR/NCRR NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- R01 AR057327/AR/NIAMS NIH HHS/United States
- K24 AR066109/AR/NIAMS NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- R03 AR083661/AR/NIAMS NIH HHS/United States
- R01 AR043727/AR/NIAMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- VAC_/Versus Arthritis/United Kingdom
- K24 AR002213/AR/NIAMS NIH HHS/United States
- DH_/Department of Health/United Kingdom
- P60 AR064464/AR/NIAMS NIH HHS/United States
- P60 AR048098/AR/NIAMS NIH HHS/United States
- R01 AR069572/AR/NIAMS NIH HHS/United States
- K24 AR002128/AR/NIAMS NIH HHS/United States

