Perceptual History Biases Are Predicted by Early Visual-Evoked Activity
- PMID: 37085319
- PMCID: PMC10218026
- DOI: 10.1523/JNEUROSCI.1451-22.2023
Perceptual History Biases Are Predicted by Early Visual-Evoked Activity
Abstract
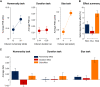
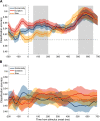
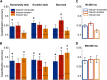
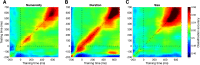
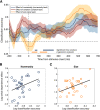
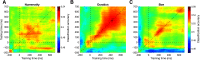
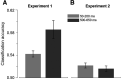
What we see in the present is affected by what we saw in the recent past. Serial dependence, a bias making a current stimulus appear more similar to a previous one, has been indeed shown to be ubiquitous in vision. At the neural level, serial dependence is accompanied by a signature of stimulus history (i.e., past stimulus information) emerging from early visual-evoked activity. However, whether this neural signature effectively reflects the behavioral bias is unclear. Here we address this question by assessing the neural (electrophysiological) and behavioral signature of stimulus history in human subjects (both male and female), in the context of numerosity, duration, and size perception. First, our results show that while the behavioral effect is task-dependent, its neural signature also reflects task-irrelevant dimensions of a past stimulus, suggesting a partial dissociation between the mechanisms mediating the encoding of stimulus history and the behavioral bias itself. Second, we show that performing a task is not a necessary condition to observe the neural signature of stimulus history, but that in the presence of an active task such a signature is significantly amplified. Finally, and more importantly, we show that the pattern of brain activity in a relatively early latency window (starting at ∼35-65 ms after stimulus onset) significantly predicts the behavioral effect. Overall, our results thus demonstrate that the encoding of past stimulus information in neural signals does indeed reflect serial dependence, and that serial dependence occurs at a relatively early level of visual processing.SIGNIFICANCE STATEMENT What we perceive is determined not only by the information reaching our sensory organs, but also by the context in which the information is embedded. What we saw in the recent past (perceptual history) can indeed modulate the perception of a current stimulus in an attractive way, a bias that is ubiquitous in vision. Here we show that this bias can be predicted by the pattern of brain activity reflecting the encoding of past stimulus information, very early after the onset of a stimulus. This in turn suggests that the integration of past and present sensory information mediating the attractive bias occurs early in the visual processing stream, and likely involves early visual cortices.
Keywords: EEG; magnitude perception; perceptual history; serial dependence.
Copyright © 2023 Fornaciai et al.
Figures



Similar articles
-
Serial dependence in visual perception: A meta-analysis and review.J Vis. 2023 Aug 1;23(8):18. doi: 10.1167/jov.23.8.18. J Vis. 2023. PMID: 37642639 Free PMC article. Review.
-
Neural Dynamics of Serial Dependence in Numerosity Perception.J Cogn Neurosci. 2020 Jan;32(1):141-154. doi: 10.1162/jocn_a_01474. Epub 2019 Sep 27. J Cogn Neurosci. 2020. PMID: 31560267
-
The effect of abstract representation and response feedback on serial dependence in numerosity perception.Atten Percept Psychophys. 2022 Jul;84(5):1651-1665. doi: 10.3758/s13414-022-02518-y. Epub 2022 May 24. Atten Percept Psychophys. 2022. PMID: 35610413
-
Serial dependence in time and numerosity perception is dimension-specific.J Vis. 2021 May 3;21(5):6. doi: 10.1167/jov.21.5.6. J Vis. 2021. PMID: 33956059 Free PMC article.
-
Serial dependence in visual perception: A review.J Vis. 2023 Jan 3;23(1):9. doi: 10.1167/jov.23.1.9. J Vis. 2023. PMID: 36648418 Free PMC article. Review.
Cited by
-
Distinct serial dependence between small and large numerosity processing.Psychol Res. 2024 Dec 31;89(1):41. doi: 10.1007/s00426-024-02071-3. Psychol Res. 2024. PMID: 39739125
-
A direct neural signature of serial dependence in working memory.Elife. 2025 Jun 23;13:RP99478. doi: 10.7554/eLife.99478. Elife. 2025. PMID: 40549432 Free PMC article.
-
The impact of task measurements on sequential dependence: a comparison between temporal reproduction and discrimination tasks.Psychol Res. 2024 Nov;88(8):2346-2359. doi: 10.1007/s00426-024-02023-x. Epub 2024 Aug 27. Psychol Res. 2024. PMID: 39190157 Free PMC article.
-
Serial dependence in visual perception: A meta-analysis and review.J Vis. 2023 Aug 1;23(8):18. doi: 10.1167/jov.23.8.18. J Vis. 2023. PMID: 37642639 Free PMC article. Review.
-
Serial dependence in face-gender classification revealed in low-beta frequency EEG.BMC Biol. 2025 Jul 8;23(1):203. doi: 10.1186/s12915-025-02289-6. BMC Biol. 2025. PMID: 40629393 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
