Development and Evaluation of a Simulation-Based Algorithm to Optimize the Planning of Interim Analyses for Clinical Trials in ALS
- PMID: 37085329
- PMCID: PMC10256133
- DOI: 10.1212/WNL.0000000000207306
Development and Evaluation of a Simulation-Based Algorithm to Optimize the Planning of Interim Analyses for Clinical Trials in ALS
Abstract
Background and objectives: Late-phase clinical trials for neurodegenerative diseases have a low probability of success. In this study, we introduce an algorithm that optimizes the planning of interim analyses for clinical trials in amyotrophic lateral sclerosis (ALS) to better use the time and resources available and minimize the exposure of patients to ineffective or harmful drugs.
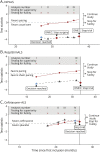
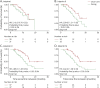
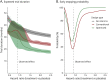
Methods: A simulation-based algorithm was developed to determine the optimal interim analysis scheme by integrating prior knowledge about the success rate of ALS clinical trials with drug-specific information obtained in early-phase studies. Interim analysis schemes were optimized by varying the number and timing of interim analyses, together with their decision rules about when to stop a trial. The algorithm was applied retrospectively to 3 clinical trials that investigated the efficacy of diaphragm pacing or ceftriaxone on survival in patients with ALS. Outcomes were additionally compared with conventional interim designs.
Results: We evaluated 183-1,351 unique interim analysis schemes for each trial. Application of the optimal designs correctly established lack of efficacy, would have concluded all studies 1.2-19.4 months earlier (reduction of 4.6%-57.7% in trial duration), and could have reduced the number of randomized patients by 1.7%-58.1%. By means of simulation, we illustrate the efficiency for other treatment scenarios. The optimized interim analysis schemes outperformed conventional interim designs in most scenarios.
Discussion: Our algorithm uses prior knowledge to determine the uncertainty of the expected treatment effect in ALS clinical trials and optimizes the planning of interim analyses. Improving futility monitoring in ALS could minimize the exposure of patients to ineffective or harmful treatments and result in significant ethical and efficiency gains.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
J.W.J. van Unnik, S. Nikolakopoulos, M.J.C. Eijkemans, J. Gonzalez-Bermejo, G. Bruneteau, C. Morelot-Panzini, L.H. van den Berg, and M.E. Cudkowicz report no disclosures relevant to the manuscript. C.J. McDermott is supported by the NIHR Sheffield Biomedical Research Center and the NIHR Research Professorship Award. T. Similowski and R.P.A. van Eijk report no disclosures relevant to the manuscript. Go to
Figures

Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Gamma aminobutyric acid (GABA) modulators for amyotrophic lateral sclerosis/motor neuron disease.Cochrane Database Syst Rev. 2017 Jan 9;1(1):CD006049. doi: 10.1002/14651858.CD006049.pub2. Cochrane Database Syst Rev. 2017. PMID: 28067943 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Trial Participation in Neurodegenerative Diseases: Barriers and Facilitators: A Systematic Review and Meta-Analysis.Neurology. 2024 Jul 9;103(1):e209503. doi: 10.1212/WNL.0000000000209503. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830181 Free PMC article.
-
Remote Monitoring of Amyotrophic Lateral Sclerosis Using Digital Health Technologies: Shifting Toward Digitalized Care and Research?Neurology. 2025 Jul 8;105(1):e213738. doi: 10.1212/WNL.0000000000213738. Epub 2025 Jun 3. Neurology. 2025. PMID: 40460337 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
