Bone Biomarkers and Subsequent Survival in Men with Hormone-sensitive Prostate Cancer: Results from the SWOG S1216 Phase 3 Trial of Androgen Deprivation Therapy with or Without Orteronel
- PMID: 37085425
- PMCID: PMC10662935
- DOI: 10.1016/j.eururo.2023.03.036
Bone Biomarkers and Subsequent Survival in Men with Hormone-sensitive Prostate Cancer: Results from the SWOG S1216 Phase 3 Trial of Androgen Deprivation Therapy with or Without Orteronel
Abstract
Background: Bone biomarkers are strongly prognostic for overall survival (OS) in men with castration-resistant prostate cancer but not fully established for hormone-sensitive prostate cancer (HSPC).
Objective: Bone biomarkers in HSPC were prospectively evaluated as part of a phase 3 study of androgen deprivation therapy ± the CYP17 inhibitor orteronel.
Design, setting, and participants: Patients were randomly divided into training (n = 316) and validation (n = 633) sets. Recursive partitioning and Cox proportional hazard models were employed.
Outcome measurements and statistical analysis: Bone resorption (C-telopeptide and pyridinoline) and bone formation markers (C-terminal collagen propeptide and bone alkaline phosphatase) were assessed from patient sera.
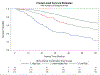
Results and limitations: Of 1279 men, 949 had evaluable baseline bone biomarkers. Optimal cutoffs were identified to define elevated levels of each of the four biomarkers (all p < 0.05) that were associated with worse OS. After adjusting for clinical risk factors in the validation set, elevated bone biomarkers were statistically significantly associated with an increased risk of death (hazard ratios ranging from 1.37 to 1.92). Recursive partitioning algorithms applied to the training set identified three risk groups (low, intermediate, and poor) with differential OS outcomes (median OS: 8.2, 5.1, and 2.1 yr, respectively) based on combinations of bone biomarkers. These results were confirmed in the validation set.
Conclusions: In men with HSPC initiating androgen deprivation therapy, bone biomarkers are strongly and independently prognostic for OS. Bone biomarker levels alone or in combination with clinical covariates identify unique subsets of men with differential OS outcomes. These results validate the clinical value of bone biomarker assessment in the HSPC state, extending bone biomarker utility beyond the castration-resistant state.
Patient summary: In men with newly diagnosed metastatic prostate cancer, high levels of bone turnover biomarkers are associated with a shorter lifespan.
Keywords: Biomarkers; Bone turnover; Hormone sensitive; Overall survival; Predictive; Prognostic; Prostate cancer.
Copyright © 2023 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement: The authors declare no potential conflicts of interest.
Figures
References
-
- Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B, Smith MR. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–36. - PubMed
-
- Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev. 2006;32 Suppl 1:23–6. - PubMed
-
- Lara PN Jr, Ely B, Quinn DI, Mack PC, Tangen C, Gertz E, Twardowski PW, Goldkorn A, Hussain M, Vogelzang NJ, Thompson IM, Van Loan MD. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J Natl Cancer Inst. 2014;106(4): dju013. - PMC - PubMed
-
- Lara PN Jr, Stadler WM, Longmate J, et al. A randomized phase II trial of the matrix metalloproteinase inhibitor BMS-275291 in hormone-refractory prostate cancer patients with bone metastases. Clinical Cancer Research 2006;12:1556–63. - PubMed