Subunit vaccines with a saponin-based adjuvant boost humoral and cellular immunity to MERS coronavirus
- PMID: 37085450
- PMCID: PMC10083212
- DOI: 10.1016/j.vaccine.2023.04.006
Subunit vaccines with a saponin-based adjuvant boost humoral and cellular immunity to MERS coronavirus
Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) outbreaks have constituted a public health issue with drastic mortality higher than 34%, necessitating the development of an effective vaccine. During MERS-CoV infection, the trimeric spike protein on the viral envelope is primarily responsible for attachment to host cellular receptor, dipeptidyl peptidase 4 (DPP4). With the goal of generating a protein-based prophylactic, we designed a subunit vaccine comprising the recombinant S1 protein with a trimerization motif (S1-Fd) and examined its immunogenicity and protective immune responses in combination with various adjuvants. We found that sera from immunized wild-type and human DPP4 transgenic mice contained S1-specific antibodies that can neutralize MERS-CoV infection in susceptible cells. Vaccination with S1-Fd protein in combination with a saponin-based QS-21 adjuvant provided long-term humoral as well as cellular immunity in mice. Our findings highlight the significance of the trimeric S1 protein in the development of MERS-CoV vaccines and offer a suitable adjuvant, QS-21, to induce robust and prolonged memory T cell response.
Keywords: Adjuvant effects; Cellular immunity; MERS-CoV neutralization; Subunit vaccine development.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ming-Fu Chang reports financial support was provided by Ministry of Science & Technology of Taiwan. Ming-Fu Chang reports a relationship with Ministry of Science & Technology of Taiwan that includes: funding grants. Ming-Fu Chang has patent NA pending to NA. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
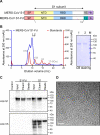

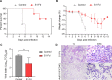
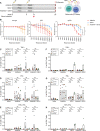
References
-
- Mou H., Raj V.S., van Kuppeveld F.J.M., Rottier P.J.M., Haagmans B.L., Bosch B.J. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol. 2013;87:9379–9383. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

