Intranasal administration of adenoviral vaccines expressing SARS-CoV-2 spike protein improves vaccine immunity in mouse models
- PMID: 37085458
- PMCID: PMC10114927
- DOI: 10.1016/j.vaccine.2023.04.020
Intranasal administration of adenoviral vaccines expressing SARS-CoV-2 spike protein improves vaccine immunity in mouse models
Abstract
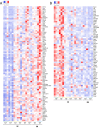
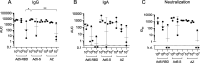
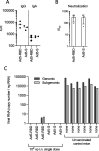
The ongoing SARS-CoV-2 pandemic is controlled but not halted by public health measures and mass vaccination strategies which have exclusively relied on intramuscular vaccines. Intranasal vaccines can prime or recruit to the respiratory epithelium mucosal immune cells capable of preventing infection. Here we report a comprehensive series of studies on this concept using various mouse models, including HLA class II-humanized transgenic strains. We found that a single intranasal (i.n.) dose of serotype-5 adenoviral vectors expressing either the receptor binding domain (Ad5-RBD) or the complete ectodomain (Ad5-S) of the SARS-CoV-2 spike protein was effective in inducing i) serum and bronchoalveolar lavage (BAL) anti-spike IgA and IgG, ii) robust SARS-CoV-2-neutralizing activity in the serum and BAL, iii) rigorous spike-directed T helper 1 cell/cytotoxic T cell immunity, and iv) protection of mice from a challenge with the SARS-CoV-2 beta variant. Intramuscular (i.m.) Ad5-RBD or Ad5-S administration did not induce serum or BAL IgA, and resulted in lower neutralizing titers in the serum. Moreover, prior immunity induced by an intramuscular mRNA vaccine could be potently enhanced and modulated towards a mucosal IgA response by an i.n. Ad5-S booster. Notably, Ad5 DNA was found in the liver or spleen after i.m. but not i.n. administration, indicating a lack of systemic spread of the vaccine vector, which has been associated with a risk of thrombotic thrombocytopenia. Unlike in otherwise genetically identical HLA-DQ6 mice, in HLA-DQ8 mice Ad5-RBD vaccine was inferior to Ad5-S, suggesting that the RBD fragment does not contain a sufficient collection of helper-T cell epitopes to constitute an optimal vaccine antigen. Our data add to previous promising preclinical results on intranasal SARS-CoV-2 vaccination and support the potential of this approach to elicit mucosal immunity for preventing transmission of SARS-CoV-2.
Keywords: Adenoviral vector; COVID-19; Intranasal vaccination; Mucosal immunity; SARS-CoV-2.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Tobias Freitag reports a relationship with Rokote Laboratories Finland Ltd that includes: employment. Kalle Saksela reports a relationship with Rokote Laboratories Finland Ltd that includes: board membership and equity or stocks. Seppo Yla-Herttuala reports a relationship with Rokote Laboratories Finland Ltd that includes: board membership and equity or stocks. Kari Alitalo reports a relationship with Rokote Laboratories Finland Ltd that includes: equity or stocks.
Figures



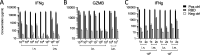


Similar articles
-
Intranasal delivery of an adenovirus-vector vaccine co-expressing a modified spike protein and a genetic adjuvant confers lasting mucosal immunity against SARS-CoV-2.Antiviral Res. 2023 Aug;216:105656. doi: 10.1016/j.antiviral.2023.105656. Epub 2023 Jun 14. Antiviral Res. 2023. PMID: 37327877 Free PMC article.
-
Intranasal SARS-CoV-2 Omicron variant vaccines elicit humoral and cellular mucosal immunity in female mice.EBioMedicine. 2024 Jul;105:105185. doi: 10.1016/j.ebiom.2024.105185. Epub 2024 Jun 7. EBioMedicine. 2024. PMID: 38848648 Free PMC article.
-
An Intranasal OMV-Based Vaccine Induces High Mucosal and Systemic Protecting Immunity Against a SARS-CoV-2 Infection.Front Immunol. 2021 Dec 17;12:781280. doi: 10.3389/fimmu.2021.781280. eCollection 2021. Front Immunol. 2021. PMID: 34987509 Free PMC article.
-
Mucosal Vaccines, Sterilizing Immunity, and the Future of SARS-CoV-2 Virulence.Viruses. 2022 Jan 19;14(2):187. doi: 10.3390/v14020187. Viruses. 2022. PMID: 35215783 Free PMC article. Review.
-
Dealing with a mucosal viral pandemic: lessons from COVID-19 vaccines.Mucosal Immunol. 2022 Apr;15(4):584-594. doi: 10.1038/s41385-022-00517-8. Epub 2022 May 3. Mucosal Immunol. 2022. PMID: 35505121 Free PMC article. Review.
Cited by
-
Evaluation of adenoviral vector Ad19a encoding RSV-F as novel vaccine against respiratory syncytial virus.NPJ Vaccines. 2024 Oct 29;9(1):205. doi: 10.1038/s41541-024-01001-z. NPJ Vaccines. 2024. PMID: 39472590 Free PMC article.
-
Next-Generation Adenoviral Vector-Based Vaccines for Severe Acute Respiratory Syndrome Coronavirus-2.Vaccines (Basel). 2025 Apr 14;13(4):406. doi: 10.3390/vaccines13040406. Vaccines (Basel). 2025. PMID: 40333307 Free PMC article. Review.
-
Multicomponent intranasal adjuvant for mucosal and durable systemic SARS-CoV-2 immunity in young and aged mice.NPJ Vaccines. 2023 Jun 29;8(1):96. doi: 10.1038/s41541-023-00691-1. NPJ Vaccines. 2023. PMID: 37386041 Free PMC article.
-
Humanized Major Histocompatibility Complex Transgenic Mouse Model Can Play a Potent Role in SARS-CoV-2 Human Leukocyte Antigen-Restricted T Cell Epitope Screening.Vaccines (Basel). 2025 Apr 15;13(4):416. doi: 10.3390/vaccines13040416. Vaccines (Basel). 2025. PMID: 40333292 Free PMC article.
References
-
- Levine-Tiefenbrun M., Yelin I., Katz R., Herzel E., Golan Z., Schreiber L., et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27:790–792. - PubMed
-
- Nanduri S., Pilishvili T., Derado G., Soe M.M., Dollard P., Wu H., et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1163–1166. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

