Igh and Igk loci use different folding principles for V gene recombination due to distinct chromosomal architectures of pro-B and pre-B cells
- PMID: 37085514
- PMCID: PMC10121685
- DOI: 10.1038/s41467-023-37994-9
Igh and Igk loci use different folding principles for V gene recombination due to distinct chromosomal architectures of pro-B and pre-B cells
Abstract
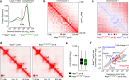
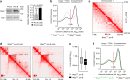
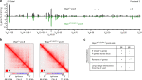
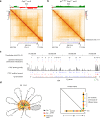
Extended loop extrusion across the immunoglobulin heavy-chain (Igh) locus facilitates VH-DJH recombination following downregulation of the cohesin-release factor Wapl by Pax5, resulting in global changes in the chromosomal architecture of pro-B cells. Here, we demonstrate that chromatin looping and VK-JK recombination at the Igk locus were insensitive to Wapl upregulation in pre-B cells. Notably, the Wapl protein was expressed at a 2.2-fold higher level in pre-B cells compared with pro-B cells, which resulted in a distinct chromosomal architecture with normal loop sizes in pre-B cells. High-resolution chromosomal contact analysis of the Igk locus identified multiple internal loops, which likely juxtapose VK and JK elements to facilitate VK-JK recombination. The higher Wapl expression in Igμ-transgenic pre-B cells prevented extended loop extrusion at the Igh locus, leading to recombination of only the 6 most 3' proximal VH genes and likely to allelic exclusion of all other VH genes in pre-B cells. These results suggest that pro-B and pre-B cells with their distinct chromosomal architectures use different chromatin folding principles for V gene recombination, thereby enabling allelic exclusion at the Igh locus, when the Igk locus is recombined.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Molecular basis for differential Igk versus Igh V(D)J joining mechanisms.Nature. 2024 Jun;630(8015):189-197. doi: 10.1038/s41586-024-07477-y. Epub 2024 May 29. Nature. 2024. PMID: 38811728 Free PMC article.
-
Wapl repression by Pax5 promotes V gene recombination by Igh loop extrusion.Nature. 2020 Aug;584(7819):142-147. doi: 10.1038/s41586-020-2454-y. Epub 2020 Jul 1. Nature. 2020. PMID: 32612238 Free PMC article.
-
Contribution of the IGCR1 regulatory element and the 3'Igh CTCF-binding elements to regulation of Igh V(D)J recombination.Proc Natl Acad Sci U S A. 2023 Jun 27;120(26):e2306564120. doi: 10.1073/pnas.2306564120. Epub 2023 Jun 20. Proc Natl Acad Sci U S A. 2023. PMID: 37339228 Free PMC article.
-
Spatial Regulation of V-(D)J Recombination at Antigen Receptor Loci.Adv Immunol. 2015;128:93-121. doi: 10.1016/bs.ai.2015.07.006. Epub 2015 Aug 13. Adv Immunol. 2015. PMID: 26477366 Review.
-
How DNA loop extrusion mediated by cohesin enables V(D)J recombination.Curr Opin Cell Biol. 2021 Jun;70:75-83. doi: 10.1016/j.ceb.2020.11.007. Epub 2021 Jan 7. Curr Opin Cell Biol. 2021. PMID: 33422934 Review.
Cited by
-
Enhancers of the PAIR4 regulatory module promote distal VH gene recombination at the Igh locus.EMBO J. 2023 Aug 1;42(15):e112741. doi: 10.15252/embj.2022112741. Epub 2023 Jun 20. EMBO J. 2023. PMID: 37337907 Free PMC article.
-
Molecular basis for differential Igk versus Igh V(D)J joining mechanisms.Nature. 2024 Jun;630(8015):189-197. doi: 10.1038/s41586-024-07477-y. Epub 2024 May 29. Nature. 2024. PMID: 38811728 Free PMC article.
-
Three-way contact analysis characterizes the higher order organization of the Tcra locus.Nucleic Acids Res. 2023 Sep 22;51(17):8987-9000. doi: 10.1093/nar/gkad641. Nucleic Acids Res. 2023. PMID: 37534534 Free PMC article.
-
ATM and 53BP1 regulate alternative end joining-mediated V(D)J recombination.Sci Adv. 2024 Aug 2;10(31):eadn4682. doi: 10.1126/sciadv.adn4682. Epub 2024 Jul 31. Sci Adv. 2024. PMID: 39083600 Free PMC article.
-
RNA processing mechanisms contribute to genome organization and stability in B cells.Oncogene. 2024 Feb;43(9):615-623. doi: 10.1038/s41388-024-02952-2. Epub 2024 Jan 29. Oncogene. 2024. PMID: 38287115 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

