Degeneration of muscle spindles in a murine model of Pompe disease
- PMID: 37085544
- PMCID: PMC10121695
- DOI: 10.1038/s41598-023-33543-y
Degeneration of muscle spindles in a murine model of Pompe disease
Erratum in
-
Author Correction: Degeneration of muscle spindles in a murine model of Pompe disease.Sci Rep. 2024 Jun 11;14(1):13394. doi: 10.1038/s41598-024-63491-0. Sci Rep. 2024. PMID: 38862583 Free PMC article. No abstract available.
Abstract
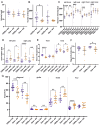
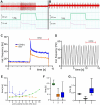
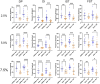
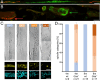
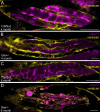
Pompe disease is a debilitating medical condition caused by a functional deficiency of lysosomal acid alpha-glucosidase (GAA). In addition to muscle weakness, people living with Pompe disease experience motor coordination deficits including an instable gait and posture. We reasoned that an impaired muscle spindle function might contribute to these deficiencies and therefore analyzed proprioception as well as muscle spindle structure and function in 4- and 8-month-old Gaa-/- mice. Gait analyses showed a reduced inter-limb and inter-paw coordination in Gaa-/- mice. Electrophysiological analyses of single-unit muscle spindle proprioceptive afferents revealed an impaired sensitivity of the dynamic and static component of the stretch response. Finally, a progressive degeneration of the sensory neuron and of the intrafusal fibers was detectable in Gaa-/- mice. We observed an increased abundance and size of lysosomes, a fragmentation of the inner and outer connective tissue capsule and a buildup of autophagic vacuoles in muscle spindles from 8-month-old Gaa-/- mice, indicating lysosomal defects and an impaired autophagocytosis. These results demonstrate a structural and functional degeneration of muscle spindles and an altered motor coordination in Gaa-/- mice. Similar changes could contribute to the impaired motor coordination in patients living with Pompe disease.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

