Diosgenin restores memory function via SPARC-driven axonal growth from the hippocampus to the PFC in Alzheimer's disease model mice
- PMID: 37085711
- PMCID: PMC10611574
- DOI: 10.1038/s41380-023-02052-9
Diosgenin restores memory function via SPARC-driven axonal growth from the hippocampus to the PFC in Alzheimer's disease model mice
Abstract
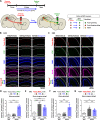
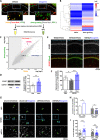
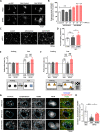
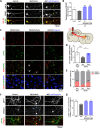
Central nervous system axons have minimal capacity to regenerate in adult brains, hindering memory recovery in Alzheimer's disease (AD). Although recent studies have shown that damaged axons sprouted in adult and AD mouse brains, long-distance axonal re-innervation to their targets has not been achieved. We selectively visualized axon-growing neurons in the neural circuit for memory formation, from the hippocampus to the prefrontal cortex, and showed that damaged axons successfully extended to their native projecting area in mouse models of AD (5XFAD) by administration of an axonal regenerative agent, diosgenin. In vivo transcriptome analysis detected the expression profile of axon-growing neurons directly isolated from the hippocampus of 5XFAD mice. Secreted protein acidic and rich in cysteine (SPARC) was the most expressed gene in axon-growing neurons. Neuron-specific overexpression of SPARC via adeno-associated virus serotype 9 delivery in the hippocampus recovered memory deficits and axonal projection to the prefrontal cortex in 5XFAD mice. DREADDs (Designer receptors exclusively activated by designer drugs) analyses revealed that SPARC overexpression-induced axonal growth in the 5XFAD mouse brain directly contributes to memory recovery. Elevated levels of SPARC on axonal membranes interact with extracellular rail-like collagen type I to promote axonal remodeling along their original tracings in primary cultured hippocampal neurons. These findings suggest that SPARC-driven axonal growth in the brain may be a promising therapeutic strategy for AD and other neurodegenerative diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Diosgenin upregulates axonal guidance partner molecules, Galectin-1 and Secernin-1.Neurosci Lett. 2024 Nov 1;842:137954. doi: 10.1016/j.neulet.2024.137954. Epub 2024 Aug 28. Neurosci Lett. 2024. PMID: 39214332
-
Axonal Regeneration Mediated by a Novel Axonal Guidance Pair, Galectin-1 and Secernin-1.Mol Neurobiol. 2023 Mar;60(3):1250-1266. doi: 10.1007/s12035-022-03125-6. Epub 2022 Nov 28. Mol Neurobiol. 2023. PMID: 36437381
-
Diosgenin restores Aβ-induced axonal degeneration by reducing the expression of heat shock cognate 70 (HSC70).Sci Rep. 2018 Aug 3;8(1):11707. doi: 10.1038/s41598-018-30102-8. Sci Rep. 2018. PMID: 30076345 Free PMC article.
-
[Development of New Therapies for Neurodegenerative Diseases via Axonal Growth].Yakugaku Zasshi. 2019;139(11):1385-1390. doi: 10.1248/yakushi.19-00147. Yakugaku Zasshi. 2019. PMID: 31685734 Review. Japanese.
-
Blood–Brain Barrier Degradation and the Implication of SPARC Protein as a Potential Therapeutic Target for Alzheimer’s Disease.In: Huang X, editor. Alzheimer’s Disease: Drug Discovery [Internet]. Brisbane (AU): Exon Publications; 2020 Dec 18. Chapter 8. In: Huang X, editor. Alzheimer’s Disease: Drug Discovery [Internet]. Brisbane (AU): Exon Publications; 2020 Dec 18. Chapter 8. PMID: 33400467 Free Books & Documents. Review.
Cited by
-
Cell-Instructive Biomaterials with Native-Like Biochemical Complexity.Annu Rev Biomed Eng. 2025 May;27(1):185-209. doi: 10.1146/annurev-bioeng-120823-020209. Epub 2025 Jan 28. Annu Rev Biomed Eng. 2025. PMID: 39874600 Review.
-
Pharmacological intervention for chronic phase of spinal cord injury.Neural Regen Res. 2025 May 1;20(5):1377-1389. doi: 10.4103/NRR.NRR-D-24-00176. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934397 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

