Advances in the optimization of central carbon metabolism in metabolic engineering
- PMID: 37085866
- PMCID: PMC10122336
- DOI: 10.1186/s12934-023-02090-6
Advances in the optimization of central carbon metabolism in metabolic engineering
Abstract
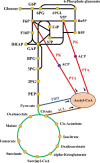
Central carbon metabolism (CCM), including glycolysis, tricarboxylic acid cycle and the pentose phosphate pathway, is the most fundamental metabolic process in the activities of living organisms that maintains normal cellular growth. CCM has been widely used in microbial metabolic engineering in recent years due to its unique regulatory role in cellular metabolism. Using yeast and Escherichia coli as the representative organisms, we summarized the metabolic engineering strategies on the optimization of CCM in eukaryotic and prokaryotic microbial chassis, such as the introduction of heterologous CCM metabolic pathways and the optimization of key enzymes or regulatory factors, to lay the groundwork for the future use of CCM optimization in metabolic engineering. Furthermore, the bottlenecks in the application of CCM optimization in metabolic engineering and future application prospects are summarized.
Keywords: Central carbon metabolism; Escherichia coli; Metabolic engineering; Metabolic pathways; Microbial chassis; Yeast.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

