Proteostasis failure exacerbates neuronal circuit dysfunction and sleep impairments in Alzheimer's disease
- PMID: 37085942
- PMCID: PMC10119020
- DOI: 10.1186/s13024-023-00617-4
Proteostasis failure exacerbates neuronal circuit dysfunction and sleep impairments in Alzheimer's disease
Abstract
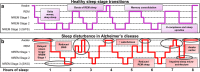
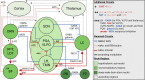
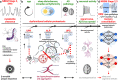
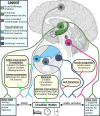
Failed proteostasis is a well-documented feature of Alzheimer's disease, particularly, reduced protein degradation and clearance. However, the contribution of failed proteostasis to neuronal circuit dysfunction is an emerging concept in neurodegenerative research and will prove critical in understanding cognitive decline. Our objective is to convey Alzheimer's disease progression with the growing evidence for a bidirectional relationship of sleep disruption and proteostasis failure. Proteostasis dysfunction and tauopathy in Alzheimer's disease disrupts neurons that regulate the sleep-wake cycle, which presents behavior as impaired slow wave and rapid eye movement sleep patterns. Subsequent sleep loss further impairs protein clearance. Sleep loss is a defined feature seen early in many neurodegenerative disorders and contributes to memory impairments in Alzheimer's disease. Canonical pathological hallmarks, β-amyloid, and tau, directly disrupt sleep, and neurodegeneration of locus coeruleus, hippocampal and hypothalamic neurons from tau proteinopathy causes disruption of the neuronal circuitry of sleep. Acting in a positive-feedback-loop, sleep loss and circadian rhythm disruption then increase spread of β-amyloid and tau, through impairments of proteasome, autophagy, unfolded protein response and glymphatic clearance. This phenomenon extends beyond β-amyloid and tau, with interactions of sleep impairment with the homeostasis of TDP-43, α-synuclein, FUS, and huntingtin proteins, implicating sleep loss as an important consideration in an array of neurodegenerative diseases and in cases of mixed neuropathology. Critically, the dynamics of this interaction in the neurodegenerative environment are not fully elucidated and are deserving of further discussion and research. Finally, we propose sleep-enhancing therapeutics as potential interventions for promoting healthy proteostasis, including β-amyloid and tau clearance, mechanistically linking these processes. With further clinical and preclinical research, we propose this dynamic interaction as a diagnostic and therapeutic framework, informing precise single- and combinatorial-treatments for Alzheimer's disease and other brain disorders.
Keywords: Alzheimer’s disease; Autophagy; Proteostasis; Sleep; Tau; Unfolded protein response; β-amyloid.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

