Activation of Tm-22 resistance is mediated by a conserved cysteine essential for tobacco mosaic virus movement
- PMID: 37086003
- PMCID: PMC10346382
- DOI: 10.1111/mpp.13318
Activation of Tm-22 resistance is mediated by a conserved cysteine essential for tobacco mosaic virus movement
Abstract
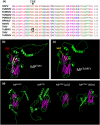
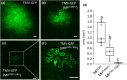
The tomato Tm-22 gene was considered to be one of the most durable resistance genes in agriculture, protecting against viruses of the Tobamovirus genus, such as tomato mosaic virus (ToMV) and tobacco mosaic virus (TMV). However, an emerging tobamovirus, tomato brown rugose fruit virus (ToBRFV), has overcome Tm-22 , damaging tomato production worldwide. Tm-22 encodes a nucleotide-binding leucine-rich repeat (NLR) class immune receptor that recognizes its effector, the tobamovirus movement protein (MP). Previously, we found that ToBRFV MP (MPToBRFV ) enabled the virus to overcome Tm-22 -mediated resistance. Yet, it was unknown how Tm-22 remained durable against other tobamoviruses, such as TMV and ToMV, for over 60 years. Here, we show that a conserved cysteine (C68) in the MP of TMV (MPTMV ) plays a dual role in Tm-22 activation and viral movement. Substitution of MPToBRFV amino acid H67 with the corresponding amino acid in MPTMV (C68) activated Tm-22 -mediated resistance. However, replacement of C68 in TMV and ToMV disabled the infectivity of both viruses. Phylogenetic and structural prediction analysis revealed that C68 is conserved among all Solanaceae-infecting tobamoviruses except ToBRFV and localizes to a predicted jelly-roll fold common to various MPs. Cell-to-cell and subcellular movement analysis showed that C68 is required for the movement of TMV by regulating the MP interaction with the endoplasmic reticulum and targeting it to plasmodesmata. The dual role of C68 in viral movement and Tm-22 immune activation could explain how TMV was unable to overcome this resistance for such a long period.
Keywords: Tm-2 2; Tobamovirus; TMV; ToBRFV; movement protein; plasmodesmata.
© 2023 The Authors. Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Figures


References
-
- Adachi, H. , Derevnina, L. & Kamoun, S. (2019) NLR singletons, pairs, and networks: evolution, assembly, and regulation of the intracellular immunoreceptor circuitry of plants. Current Opinion in Plant Biology, 50, 121–131. - PubMed
-
- Bi, G. , Su, M. , Li, N. , Liang, Y. , Dang, S. , Xu, J. et al. (2021) The ZAR1 resistosome is a calcium‐permeable channel triggering plant immune signaling. Cell, 184, 3528–3541. - PubMed
-
- Broadbent, L. (1976) Epidemiology and control of tomato mosaic virus. Annual Review of Phytopathology, 14, 75–96.

