Causal inference in survival analysis using longitudinal observational data: Sequential trials and marginal structural models
- PMID: 37086186
- PMCID: PMC7614580
- DOI: 10.1002/sim.9718
Causal inference in survival analysis using longitudinal observational data: Sequential trials and marginal structural models
Abstract
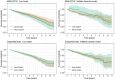
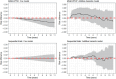
Longitudinal observational data on patients can be used to investigate causal effects of time-varying treatments on time-to-event outcomes. Several methods have been developed for estimating such effects by controlling for the time-dependent confounding that typically occurs. The most commonly used is marginal structural models (MSM) estimated using inverse probability of treatment weights (IPTW) (MSM-IPTW). An alternative, the sequential trials approach, is increasingly popular, and involves creating a sequence of "trials" from new time origins and comparing treatment initiators and non-initiators. Individuals are censored when they deviate from their treatment assignment at the start of each "trial" (initiator or noninitiator), which is accounted for using inverse probability of censoring weights. The analysis uses data combined across trials. We show that the sequential trials approach can estimate the parameters of a particular MSM. The causal estimand that we focus on is the marginal risk difference between the sustained treatment strategies of "always treat" vs "never treat." We compare how the sequential trials approach and MSM-IPTW estimate this estimand, and discuss their assumptions and how data are used differently. The performance of the two approaches is compared in a simulation study. The sequential trials approach, which tends to involve less extreme weights than MSM-IPTW, results in greater efficiency for estimating the marginal risk difference at most follow-up times, but this can, in certain scenarios, be reversed at later time points and relies on modelling assumptions. We apply the methods to longitudinal observational data from the UK Cystic Fibrosis Registry to estimate the effect of dornase alfa on survival.
Keywords: cystic fibrosis; inverse probability weighting; marginal structural model; registries; sequential trials; survival; target trials; time-dependent confounding.
© 2023 The Authors. Statistics in Medicine published by John Wiley & Sons Ltd.
Figures
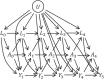
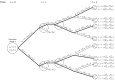
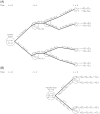


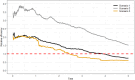

References
-
- Robins J, Hernán M, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550‐560. - PubMed
-
- Hernán M, Brumback B, Robins J. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV‐positive men. Epidemiology. 2000;11:561‐569. - PubMed
-
- Clare P, Dobbins T, Mattick R. Causal models adjusting for time‐varying confounding – a systematic review of the literature. Int J Epidemiol. 2019;48:254‐265. - PubMed
-
- Gran J, Røysland K, Wolbers M, et al. A sequential Cox approach for estimating the causal effect of treatment in the presence of time‐dependent confounding applied to data from the Swiss HIV cohort study. Stat Med. 2010;29:2757‐2768. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
