Beyond Skin Rash: Alpelisib-Induced Anaphylactic Reactions
- PMID: 37086483
- PMCID: PMC10322120
- DOI: 10.1093/oncolo/oyad092
Beyond Skin Rash: Alpelisib-Induced Anaphylactic Reactions
Abstract
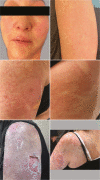
Alpelisib is a specific oral PI3K inhibitor used combined with fulvestrant for the treatment of patients with HR+/HER2-/PIK3CA-mutated metastatic breast cancer. Adverse drug reactions with alpelisib are common, including hyperglycemia and rash. Here we describe extraordinary and life-threatening reactions beyond skin rash in two patients with progressive PIK3CA-mutated metastatic cancer in whom alpelisib was initiated. Case-A (vaginal cancer): After 10 days on treatment, she developed dry eyes, generalized rash and itching. Alpelisib was interrupted and symptomatic treatment initiated. Because of an initial tumor response, a rechallenge was done. Ninety minutes after a reduced dose of alpelisib, she developed an anaphylactic reaction with angioedema, hypotension, and skin rash. Case-B (breast cancer): After 11 days on treatment, she developed skin rash and alpelisib was interrupted. At re-initiation, she felt tingles in her face and ears and some skin erythema. Given the mild rash, a second rechallenge with premedication was performed. Ninety minutes after a reduced dose of alpelisib, she developed a type-1 allergic reaction with angioedema, tingles, and skin rash. In both cases, a type-1 allergic reaction was diagnosed and symptomatic treatment was initiated, alpelisib was permanently discontinued and the patients fully recovered the next week(s). This report underlines the critical importance to consider type-I allergic reactions in the differential diagnosis in cases of rash associated with alpelisib. Even if a reaction develops after days on treatment, a type-I allergic reaction cannot be excluded. A rechallenge can be dangerous and should always be well contemplated or even avoided.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Laurien Zeverijn, Birgit Geurts, and Gijs de Wit have received grants and non-financial support from Novartis in the context of the Drug Rediscovery Protocol, not related to the research, authorship, and/or publication of this article. The other authors (Tim Schutte, Marleen Kok, and Frans Opdam) declare no competing financial interests or non-financial interests, except from the review of the manuscript by Novartis, as part of the agreement between Novartis and the DRUP-trial investigators in accordance with the Drug Rediscovery Trial Protocol. No significant changes to the content of the manuscript were suggested. The suggested changes of Novartis and versions of the manuscript before and after their review are available upon reasonable request from the corresponding author.
Figures

References
-
- Agency EM. Summary of Product Characteristics - Alpelisib (Piqray). https://www.ema.europa.eu/en/documents/product-information/piqray-epar-p....
-
- Mayer IA, Prat A, Egle D, et al. . A phase II randomized study of neoadjuvant letrozole plus alpelisib for hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (NEO-ORB). Clin Cancer Res. 2019;25(10):2975-2987. 10.1158/1078-0432.CCR-18-3160. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

