Omicron BA.1-specific T-cell responses in adults vaccinated with CoronaVac or BNT162b2 in Hong Kong: an observational cohort study
- PMID: 37086735
- PMCID: PMC10115591
- DOI: 10.1016/S2666-5247(23)00006-X
Omicron BA.1-specific T-cell responses in adults vaccinated with CoronaVac or BNT162b2 in Hong Kong: an observational cohort study
Abstract
Background: The primary aim of using vaccines in public health responses to SARS-CoV-2 variants of concern is to reduce incidence of severe disease, for which T-cell responses are essential. There is a paucity of data on vaccine-induced T-cell immunity to omicron (B.1.1.529). We aimed to compare SARS-CoV-2 omicron BA.1-specific T-cell responses in adults vaccinated with CoronaVac or BNT162b2.
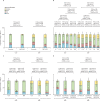
Methods: For this observational cohort, we recruited adults (aged ≥18 years) from three vaccination centres in Hong Kong. We included participants from four cohorts (cohort 1: participants who received two doses of either BNT162b2 or CoronaVac, cohort 2: participants who received two doses and a booster, cohort 3: participants who received two doses and a booster and had a breakthrough omicron infection, and cohort 4: participants who had a previous non-omicron infection and subsequently received one dose of vaccine). People with confirmed history of COVID-19 at recruitment were excluded from cohort 1 and cohort 2. We collected blood samples before vaccination (for cohort 1 and 2), 1-month following vaccination (for all cohorts), and during convalescence for cohort 3 and 4) and determined the proportion of IFNγ+CD4+ and IFNγ+CD8+ T cells in peripheral blood against SARS-CoV-2 using flow cytometry with peptide pools of SARS-CoV-2 wild type or omicron BA.1. The primary outcome was proportion of CD4+ and CD8+ T cells against SARS-CoV-2 1 month after exposure (ie, vaccination or breakthrough infection).
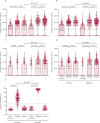
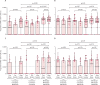
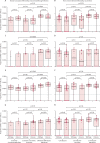
Findings: Overall, between May 21, 2020, and Aug 31, 2021, we recruited 659 participants (231 [35%] men and 428 [65%] women). Of these participants, 428 were included in cohort 1 (214 [50%] received BNT162b2 and 214 [50%] received CoronaVac); 127 in cohort 2 (48 [38%] received all BNT162b2, 40 [31%] received all CoronaVac, and 39 [31%] received two CoronaVac and a booster with BNT162b2); 58 in cohort 3, and 46 in cohort 4 (16 [35%] received CoronaVac and 30 [65%] received BNT162b2). Vaccine-induced T-cell responses to the wild-type and omicron BA.1 variants were generally similar in adults receiving two doses of either CoronaVac (CD4+ cells p=0·33; CD8+ cells p=0·70) or BNT162b2 (CD4+ cells p=0·28; CD8+ cells p=1·0). Using a peptide pool of all structural proteins for stimulation, BNT162b2 induced a higher median frequency of omicron-specific CD4+ T cells in adults younger than 60 years (CD4+ cells 0·012% vs 0·010%, p=0·031; CD8+ cells 0·003% vs 0·000%, p=0·055) and omicron-specific CD8+ T cells in people aged 60 years or older (CD4+ cells 0·015% vs 0·006%, p=0·0070; CD8+ cells 0·007% vs 0·000%, p=0·035). A booster dose of either BNT162b2 or CoronaVac after two doses of CoronaVac boosted waning T-cell responses, but T-cell responses did not exceed those at 1 month after the second dose (CoronaVac CD4+ p=0·41, CD8+ p=0·79; BNT162b2 CD4+ p=0·70 CD8+ p=0·80).
Interpretation: The evidence that mRNA and inactivated vaccines based on the ancestral SARS-CoV-2 virus elicited T-cell responses to SARS-CoV-2 omicron variants might explain the high observed vaccine effectiveness against severe COVID-19 shown by both types of vaccine, despite great differences in neutralising antibody responses. The use of either vaccine can be considered if the primary aim is to reduce severity and death caused by the new omicron subvariants; however, BNT162b2 is preferable for adults older than 60 years.
Funding: The Health and Medical Research Fund Commissioned Research on the Novel Coronavirus Disease and S H Ho Foundation.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Similar articles
-
Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA.2 in Hong Kong: a prospective cohort study.Lancet Infect Dis. 2023 Apr;23(4):421-434. doi: 10.1016/S1473-3099(22)00732-0. Epub 2022 Dec 12. Lancet Infect Dis. 2023. PMID: 36521506 Free PMC article.
-
Comparative antibody and cell-mediated immune responses, reactogenicity, and efficacy of homologous and heterologous boosting with CoronaVac and BNT162b2 (Cobovax): an open-label, randomised trial.Lancet Microbe. 2023 Sep;4(9):e670-e682. doi: 10.1016/S2666-5247(23)00216-1. Epub 2023 Aug 4. Lancet Microbe. 2023. PMID: 37549680 Free PMC article. Clinical Trial.
-
Vaccine effectiveness of BNT162b2 and CoronaVac against SARS-CoV-2 omicron infection and related hospital admission among people with substance use disorder in Hong Kong: a matched case-control study.Lancet Psychiatry. 2023 Jun;10(6):403-413. doi: 10.1016/S2215-0366(23)00111-6. Epub 2023 May 1. Lancet Psychiatry. 2023. PMID: 37141907 Free PMC article.
-
Safety and effectiveness of vaccines against COVID-19 in children aged 5-11 years: a systematic review and meta-analysis.Lancet Child Adolesc Health. 2023 Jun;7(6):379-391. doi: 10.1016/S2352-4642(23)00078-0. Epub 2023 Apr 18. Lancet Child Adolesc Health. 2023. PMID: 37084750 Free PMC article.
-
SARS-CoV-2 Omicron variants: burden of disease, impact on vaccine effectiveness and need for variant-adapted vaccines.Front Immunol. 2023 May 23;14:1130539. doi: 10.3389/fimmu.2023.1130539. eCollection 2023. Front Immunol. 2023. PMID: 37287979 Free PMC article. Review.
Cited by
-
The immunological impact of revaccination in a hybrid-immune world.Front Immunol. 2025 Jun 9;16:1588259. doi: 10.3389/fimmu.2025.1588259. eCollection 2025. Front Immunol. 2025. PMID: 40552302 Free PMC article. Review.
-
The role of the gut microbiota in regulating responses to vaccination: current knowledge and future directions.FEBS J. 2025 Mar;292(6):1480-1499. doi: 10.1111/febs.17241. Epub 2024 Aug 5. FEBS J. 2025. PMID: 39102299 Free PMC article. Review.
-
Global Emergence of SARS-CoV2 Infection and Scientific Interventions to Contain its Spread.Curr Protein Pept Sci. 2024;25(4):307-325. doi: 10.2174/0113892037274719231212044235. Curr Protein Pept Sci. 2024. PMID: 38265408 Review.
-
A quest for universal anti-SARS-CoV-2 T cell assay: systematic review, meta-analysis, and experimental validation.NPJ Vaccines. 2024 Jan 2;9(1):3. doi: 10.1038/s41541-023-00794-9. NPJ Vaccines. 2024. PMID: 38167915 Free PMC article.
-
Cellular immune breadth of an Omicron-specific, self-amplifying monovalent mRNA vaccine booster for COVID-19.NPJ Vaccines. 2025 Mar 1;10(1):42. doi: 10.1038/s41541-025-01076-2. NPJ Vaccines. 2025. PMID: 40025095 Free PMC article.
References
-
- WHO Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Nov 26, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1....
-
- Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence—25 US jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132–138. - PMC - PubMed
-
- WHO COVID-19 vaccines. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19...
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

