Transplantation Referral Patterns for Patients with Newly Diagnosed Higher-Risk Myelodysplastic Syndromes and Acute Myeloid Leukemia at Academic and Community Sites in the Connect® Myeloid Disease Registry: Potential Barriers to Care
- PMID: 37086851
- PMCID: PMC11104018
- DOI: 10.1016/j.jtct.2023.04.011
Transplantation Referral Patterns for Patients with Newly Diagnosed Higher-Risk Myelodysplastic Syndromes and Acute Myeloid Leukemia at Academic and Community Sites in the Connect® Myeloid Disease Registry: Potential Barriers to Care
Abstract
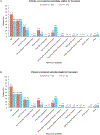
Hematopoietic stem cell transplantation (HCT) is indicated for patients with higher-risk (HR) myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Age, performance status, patient frailty, comorbidities, and nonclinical factors (eg, cost, distance to site) are all recognized as important clinical factors that can influence HCT referral patterns and patient outcomes; however, the proportion of eligible patients referred for HCT in routine clinical practice is largely unknown. This study aimed to assess patterns of consideration for HCT among patients with HR-MDS and AML enrolled in the Connect® Myeloid Disease Registry at community/government (CO/GOV)- or academic (AC)-based sites, as well as to identify factors associated with rates of transplantation referral. We assessed patterns of consideration for and completion of HCT in patients with HR-MDS and AML enrolled between December 12, 2013, and March 6, 2020, in the Connect Myeloid Disease Registry at 164 CO/GOV and AC sites. Registry sites recorded whether patients were considered for transplantation at baseline and at each follow-up visit. The following answers were possible: "considered potentially eligible," "not considered potentially eligible," or "not assessed." Sites also recorded whether patients subsequently underwent HCT at each follow-up visit. Rates of consideration for HCT between CO/GOV and AC sites were compared using multivariable logistic regression analysis with covariates for age and comorbidity. Among the 778 patients with HR-MDS or AML enrolled in the Connect Myeloid Disease Registry, patients at CO/GOV sites were less likely to be considered potentially eligible for HCT than patients at AC sites (27.9% versus 43.9%; P < .0001). Multivariable logistic regression analysis with factors for age (<65 versus ≥65 years) and ACE-27 comorbidity grade (<2 versus ≥2) showed that patients at CO/GOV sites were significantly less likely than those at AC sites to be considered potentially eligible for HCT (odds ratio, 1.6, 95% confidence interval, 1.1 to 2.4; P = .0155). Among patients considered eligible for HCT, 45.1% (65 of 144) of those at CO/GOV sites and 35.7% (41 of 115) of those at AC sites underwent transplantation (P = .12). Approximately one-half of all patients at CO/GOV (50.1%) and AC (45.4%) sites were not considered potentially eligible for HCT; the most common reasons were age at CO/GOV sites (71.5%) and comorbidities at AC sites (52.1%). Across all sites, 17.4% of patients were reported as not assessed (and thus not considered) for HCT by their treating physician (20.7% at CO/GOV sites and 10.7% at AC sites; P = .0005). These findings suggest that many patients with HR-MDS and AML who may be candidates for HCT are not receiving assessment or consideration for transplantation in clinical practice. In addition, treatment at CO/GOV sites and age remain significant barriers to ensuring that all potentially eligible patients are assessed for HCT.
Keywords: Acute myeloid leukemia; Bone marrow transplantation; Clinical practice; Hematologic malignancies; Management and treatment; Myelodysplastic syndromes.
Copyright © 2023 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Connect MDS/AML: design of the myelodysplastic syndromes and acute myeloid leukemia disease registry, a prospective observational cohort study.BMC Cancer. 2016 Aug 19;16:652. doi: 10.1186/s12885-016-2710-6. BMC Cancer. 2016. PMID: 27538433 Free PMC article.
-
TP53 Mutations Are Associated with Increased Infections and Reduced Hematopoietic Cell Transplantation Rates in Myelodysplastic Syndrome and Acute Myeloid Leukemia.Transplant Cell Ther. 2023 Jun;29(6):390.e1-390.e10. doi: 10.1016/j.jtct.2023.03.008. Epub 2023 Mar 9. Transplant Cell Ther. 2023. PMID: 36906277
-
Acute myeloid leukemia or myelodysplastic syndrome with chromosome 17 abnormalities and long-term outcomes with or without hematopoietic stem cell transplantation.Leuk Res. 2020 Aug;95:106402. doi: 10.1016/j.leukres.2020.106402. Epub 2020 Jun 18. Leuk Res. 2020. PMID: 32590108
-
Hematopoietic Cell Transplantation for Myelodysplastic Syndromes.J Oncol Pract. 2016 Sep;12(9):786-92. doi: 10.1200/JOP.2016.015214. J Oncol Pract. 2016. PMID: 27621329 Review.
-
Allogeneic Transplantation for Older Adults.Adv Exp Med Biol. 2025;1475:9-40. doi: 10.1007/978-3-031-84988-6_2. Adv Exp Med Biol. 2025. PMID: 40488822 Review.
Cited by
-
Applying Implementation Science in the Field of Transplant and Cellular Therapy.Transplant Cell Ther. 2024 Sep;30(9):864-875. doi: 10.1016/j.jtct.2024.06.018. Epub 2024 Jun 21. Transplant Cell Ther. 2024. PMID: 38909780 Review.
-
Allogeneic hematopoietic cell transplantation in elderly patients with myelodysplastic syndromes: Considerations and challenges.Semin Hematol. 2024 Dec;61(6):420-430. doi: 10.1053/j.seminhematol.2024.10.004. Epub 2024 Oct 17. Semin Hematol. 2024. PMID: 39523201 Review.
-
Role of Geriatric Assessment in Hematopoietic Stem Cell Transplant and Cellular Therapies.Curr Treat Options Oncol. 2025 May;26(5):348-359. doi: 10.1007/s11864-025-01316-6. Epub 2025 Apr 10. Curr Treat Options Oncol. 2025. PMID: 40208382 Review.
References
-
- Runde V, de Witte T, Arnold R, et al. Bone marrow transplantation from HLA-identical siblings as first-line treatment in patients with myelodysplastic syndromes: early transplantation is associated with improved outcome. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;21:255–261. 10.1038/sj.bmt.1701084. - DOI - PubMed
-
- Koreth J, Pidala J, Perez WS, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31:2662–2670. 10.1200/JCO.2012.46.8652. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous