Lactobacillus crispatus-loaded electrospun fibers yield viable and metabolically active bacteria that kill Gardnerella in vitro
- PMID: 37086869
- PMCID: PMC10192109
- DOI: 10.1016/j.ejpb.2023.04.011
Lactobacillus crispatus-loaded electrospun fibers yield viable and metabolically active bacteria that kill Gardnerella in vitro
Abstract
Bacterial vaginosis (BV) is a common condition that affects one-third of women worldwide. BV is characterized by low levels of healthy lactobacilli and an overgrowth of common anaerobes such as Gardnerella. Antibiotics for BV are administered orally or vaginally; however, approximately half of those treated will experience recurrence within 6 months. Lactobacillus crispatus present at high levels has been associated with positive health outcomes. To address the high recurrence rates following BV treatment, beneficial bacteria have been considered as an alternative or adjunct modality. This study aimed to establish proof-of-concept for a new long-acting delivery vehicle for L. crispatus. Here, it is shown that polyethylene oxide (PEO) fibers loaded with L. crispatus can be electrospun with poly(lactic-co-glycolic acid) (PLGA) fibers (ratio 1:1), and that this construct later releases L. crispatus as metabolically viable bacteria capable of lactic acid production and anti-Gardnerella activity. Probiotic-containing fibers were serially cultured in MRS (deMan, Rogosa, Sharpe) broth with daily media replacement and found to yield viable L. crispatus for at least 7 days. Lactic acid levels and corresponding pH values generally corresponded with levels of L. crispatus cultured from the fibers and strongly support the conclusion that fibers yield viable L. crispatus that is metabolically active. Cultures of L. crispatus-loaded fibers limited the growth of Gardnerella in a dilution-dependent manner during in vitro assays in the presence of cultured vaginal epithelial cells, demonstrating bactericidal potential. Exposure of VK2/E6E7 cells to L. crispatus-loaded fibers resulted in minimal loss of viability relative to untreated cells. Altogether, these data provide proof-of-concept for electrospun fibers as a candidate delivery vehicle for application of vaginal probiotics in a long-acting form.
Keywords: Bacterial vaginosis; Electrospinning; Gardnerella; Lactobacillus crispatus; Nanofibers; Poly(lactic-co-glycolic acid); Polyethylene oxide.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

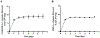
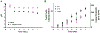


Similar articles
-
Preclinical validation of electrospun fibers to achieve vaginal colonization by Lactobacillus crispatus.Front Bacteriol. 2025;4:1562077. doi: 10.3389/fbrio.2025.1562077. Epub 2025 Apr 16. Front Bacteriol. 2025. PMID: 40831599 Free PMC article.
-
Mesh and layered electrospun fiber architectures as vehicles for Lactobacillus acidophilus and Lactobacillus crispatus intended for vaginal delivery.Biomater Adv. 2023 Nov;154:213614. doi: 10.1016/j.bioadv.2023.213614. Epub 2023 Aug 29. Biomater Adv. 2023. PMID: 37659215 Free PMC article.
-
Sustained dual delivery of metronidazole and viable Lactobacillus crispatus from 3D-printed silicone shells.Biomater Adv. 2024 Dec;165:214005. doi: 10.1016/j.bioadv.2024.214005. Epub 2024 Aug 26. Biomater Adv. 2024. PMID: 39208497
-
Probiotics for the treatment of women with bacterial vaginosis.Clin Microbiol Infect. 2007 Jul;13(7):657-64. doi: 10.1111/j.1469-0691.2007.01688.x. Clin Microbiol Infect. 2007. PMID: 17633390 Review.
-
Vaginal microbiome.Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English.
Cited by
-
Rapid-dissolving electrospun nanofibers for intra-vaginal antibiotic or probiotic delivery.Eur J Pharm Biopharm. 2023 Sep;190:81-93. doi: 10.1016/j.ejpb.2023.07.009. Epub 2023 Jul 19. Eur J Pharm Biopharm. 2023. PMID: 37479065 Free PMC article.
-
Preclinical validation of electrospun fibers to achieve vaginal colonization by Lactobacillus crispatus.Front Bacteriol. 2025;4:1562077. doi: 10.3389/fbrio.2025.1562077. Epub 2025 Apr 16. Front Bacteriol. 2025. PMID: 40831599 Free PMC article.
-
Mesh and layered electrospun fiber architectures as vehicles for Lactobacillus acidophilus and Lactobacillus crispatus intended for vaginal delivery.Biomater Adv. 2023 Nov;154:213614. doi: 10.1016/j.bioadv.2023.213614. Epub 2023 Aug 29. Biomater Adv. 2023. PMID: 37659215 Free PMC article.
-
Sexual transmission of urogenital bacteria: whole metagenome sequencing evidence from a sexual network study.mSphere. 2024 Mar 26;9(3):e0003024. doi: 10.1128/msphere.00030-24. Epub 2024 Feb 15. mSphere. 2024. PMID: 38358269 Free PMC article.
-
Electrospun nanofibers: Focus on local therapeutic delivery targeting infectious disease.J Drug Deliv Sci Technol. 2025 Feb;104:106520. doi: 10.1016/j.jddst.2024.106520. Epub 2024 Dec 20. J Drug Deliv Sci Technol. 2025. PMID: 39802685
References
-
- Vicente R-L, Roger LC, and Jack DS, Emerging Role of Lactobacilli in the Control and Maintenance of the Vaginal Bacterial Microflora. Reviews of Infectious Diseases, 1990. 12(5): p. 856–872. - PubMed
-
- Allsworth JE and Peipert JF, Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol, 2007. 109(1): p. 114–20. - PubMed
-
- Mastromarino P, Vitali B, and Mosca L, Bacterial vaginosis: a review on clinical trials with probiotics. New Microbiol, 2013. 36(3): p. 229–38. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

