A multi-site trial of an electronic health integrated physical activity promotion intervention in breast and endometrial cancers survivors: MyActivity study protocol
- PMID: 37086916
- PMCID: PMC10413251
- DOI: 10.1016/j.cct.2023.107187
A multi-site trial of an electronic health integrated physical activity promotion intervention in breast and endometrial cancers survivors: MyActivity study protocol
Abstract
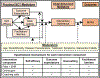
Despite the known benefits of moderate-to-vigorous physical activity (MVPA) for breast and endometrial cancer survivors, most are insufficiently active, interventions response is heterogeneous, and MVPA programming integration into cancer care is limited. A stepped care approach, in which the least resource-intensive intervention is delivered first and additional components are added based on individual response, is one strategy to enhance uptake of physical activity programming. However, the most effective intervention augmentation strategies are unknown. In this singly randomized trial of post-treatment, inactive breast and endometrial cancer survivors (n = 323), participants receive a minimal intervention including a Fitbit linked with their clinic's patient portal and, in turn, the electronic health record (EHR) with weekly feedback delivered via the portal. MVPA progress summaries are sent to participants' oncology team via the EHR. MVPA adherence is evaluated at 4, 8, 12, 16 and 20 weeks; non-responders (those meeting ≤80% of the MVPA goal over previous 4 weeks) at each timepoint are randomized once for the remainder of the 24-week intervention to one of two "step-up" conditions: (1) online gym or (2) coaching calls, while responders continue with the minimal Fitbit+EHR intervention. The primary outcome is ActiGraph-measured MVPA at 24 and 48 weeks. Secondary outcomes include symptom burden and functional performance at 24 and 48 weeks. This trial will inform development of an effective, scalable, and tailored intervention for survivors by identifying non-responders and providing them with the intervention augmentations necessary to increase MVPA and improve health outcomes. Clinical Trials Registration # NCT04262180.
Keywords: Adaptive intervention; Breast cancer; Digital health; Endometrial cancer; Physical activity; cancer survivorship.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Amye J. Tevaarwerk Epic Systems- family member.
Figures
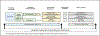
References
-
- Miller KD, et al. , Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin, 2022. - PubMed
-
- Bellizzi KM, et al. , Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol, 2005. 23(34): p. 8884–93. - PubMed
-
- Courneya KS, et al. , Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecologic Oncology, 2005. 97(2): p. 422–430. - PubMed
-
- Courneya KS, Katzmarzyk PT, and Bacon E, Physical activity and obesity in Canadian cancer survivors: population-based estimates from the 2005 Canadian Community Health Survey. Cancer, 2008. 112(11): p. 2475–82. - PubMed
-
- Blanchard CM, et al. , Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol, 2008. 26(13): p. 2198–204. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

