Matricellular proteins in atherosclerosis development
- PMID: 37086928
- PMCID: PMC10225360
- DOI: 10.1016/j.matbio.2023.04.003
Matricellular proteins in atherosclerosis development
Abstract
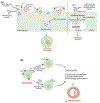
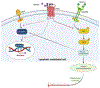
The extracellular matrix (ECM) is an intricate network composed of various multi-domain macromolecules like collagen, proteoglycans, and fibronectin, etc., that form a structurally stable composite, contributing to the mechanical properties of tissue. However, matricellular proteins are non-structural, secretory extracellular matrix proteins, which modulate various cellular functions via interacting with cell surface receptors, proteases, hormones, and cell-matrix. They play essential roles in maintaining tissue homeostasis by regulating cell differentiation, proliferation, adhesion, migration, and several signal transduction pathways. Matricellular proteins display a broad functionality regulated by their multiple structural domains and their ability to interact with different extracellular substrates and/or cell surface receptors. The expression of these proteins is low in adults, however, gets upregulated following injuries, inflammation, and during tumor growth. The marked elevation in the expression of these proteins during atherosclerosis suggests a positive association between their expression and atherosclerotic lesion formation. The role of matricellular proteins in atherosclerosis development has remained an area of research interest in the last two decades and studies revealed these proteins as important players in governing vascular function, remodeling, and plaque formation. Despite extensive research, many aspects of the matrix protein biology in atherosclerosis are still unknown and future studies are required to investigate whether targeting pathways stimulated by these proteins represent viable therapeutic approaches for patients with atherosclerotic vascular diseases. This review summarizes the characteristics of distinct matricellular proteins, discusses the available literature on the involvement of matrix proteins in the pathogenesis of atherosclerosis and suggests new avenues for future research.
Keywords: Atherosclerosis; Inflammation; Macrophages; Matricellular proteins; Vascular smooth muscle cells.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All authors declare that they have no competing interests.
Figures

References
-
- Dahlof B, Cardiovascular disease risk factors: epidemiology and risk assessment, Am J Cardiol 105(1 Suppl) (2010) 3A–9A. - PubMed
-
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS, Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association, Circulation 145(8) (2022) e153–e639. - PubMed
-
- Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, Fowkes FGR, Fowkes FJI, Rudan I, Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study, Lancet Glob Health 8(5) (2020) e721–e729. - PubMed
-
- Tucker WD, Arora Y, Mahajan K, Anatomy, Blood Vessels, StatPearls, Treasure Island (FL), 2022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

